SECTION 1. IDENTIFICATION
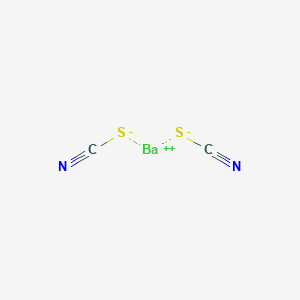
Product Name: Barium Thiocyanate
Product Number: All applicable American Elements product codes, e.g. BA-THCY-02-C.AHYD
CAS #: 2092-17-3
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Statement of Hazard: Irritant, Respiratory irritant, Toxic
Acute Health Hazard: Irritant to eyes, skin, mucous membranes and respiratory system.
May be toxic by ingestion, harmful by skin absorption and inhalation.
Chronic Health Hazard: Not Available
HMIS Rating: H: 3 F: 1 P: 0
NFPA Rating: H: 3 F: 1 P: 0
To the best of our knowledge, the toxicological properties of this chemical have not been
thoroughly investigated. Use appropriate procedures and precautions to prevent or minimize
exposure.

Pictogram:
Signal Word: Danger
Hazard Statement(s): H301 Toxic if swallowed.
H312 Harmful in contact with skin.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H332 Harmful if inhaled.
H335 May cause respiratory irritation.
Precautionary Statement(s): P260 Do not breathe dust/fume/gas/mist/vapors/spray.
P264 Wash skin thoroughly after handling.
P270 Do not eat, drink, or smoke when using this product.
P280 Wear protective gloves/protective clothing/eye protection/face
protection.
P301+P310 IF SWALLOWED: Immediately call a POISON CENTER or
doctor/physician.
P302+P352 IF ON SKIN: wash with plenty of soap and water.
P304+P340 IF INHALED: Remove victim to fresh air and Keep at rest in a
position comfortable for breathing.
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several
minutes. Remove contact lenses, if present and easy to do. Continue
rinsing.
P332+P313 IF SKIN irritation occurs: Get medical advice/attention.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Name: Barium thiocyanate
CAS Number: 2092-17-3
MDL Number: MFCD00014179
EINECS Number: 218-245-9
Beilstein Registry Number: Not Available
Molecular Formula: Ba(SCN)2.3H2O
Molecular Weight: 271.51
Content: As specified in product name.
SECTION 4. FIRST AID MEASURES
Eye Contact: Flush eyes with large amounts of water for fifteen minutes. Separate eyelids with fingers. If irritation persists, seek medical attention.
Skin Contact: Wash skin with soap and water. If irritation persists, seek medical attention.
Ingestion: Do not induce vomiting. Seek medical attention.
Inhalation: Move to a fresh air environment. Contact a physician if breathing becomes difficult.
SECTION 5. FIREFIGHTING MEASURES
Flash Point (ºC): Not Available
Explosion Limits: Not Available
Auto Ignition
Temperature (ºC): Not Available
Extinguishing Media: Carbon dioxide, dry chemical powder, alcohol-resistant foam, or water spray
Protective Equipment: Wear self-contained respirator and fully protective impervious suit.
Specific Hazards: May emit hazardous fumes under fire conditions.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal Protection: Wear a self-contained breathing apparatus, rubber boots and gloves,
and disposable coveralls. Dispose of coveralls after use. Remove from
ignition sources if safe to do so. Follow emergency response plan and
contact proper authorities if needed.
Keep unprotected persons away.
Environmental Protection: Keep spills out of sewers and bodies of water. Dike and contain the
spill with inert material. Absorb on sand, vermiculite or diatomite.
Transfer material to a container for disposal or recovery. Ventilate area
and wash spill site after material pickup is complete.
SECTION 7. HANDLING AND STORAGE
Handling and Storage: Avoid breathing dust, vapor, mist or gas. Avoid contact with skin and eyes. Avoid prolonged or repeated exposure. Use only in a chemical fume hood. Open and handle container with care. Keep ignition sources away. Store in a tightly closed container in a dry, well-ventilated place.
Sensitivities: Not Available
Storage Temperature (ºC): 15 to 30
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Engineering Controls: Use product in a well ventilated area or under a fume hood. Use proper
lab equipment while handling this product. Keep away from
incompatible materials for possible risk of hazardous reaction.
Eye Protection: Wear appropriate protective eyeglass or chemical safety goggles. Make
sure that there is an eyewash station in your vicinity.
Skin Protection: Wear impervious gloves and protective clothing.
Respiratory Protection: Use a NIOSH approved respirator when exposure limits are exceeded or
if irritation or other symptoms are experienced.
Exposure Limits: Country Source Type Value
USA ACGIH TWA Not Available
USA OSHA STEL Not Available
USA OSHA PEL Not Available
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance: Solid
Odor: Not Available
Odor Threshold: Not Available
Flash Point (ºC): Not Available
Auto Ignition
Temperature (ºC): Not Available
UEL % by Volume: Not Available
LEL % by Volume: Not Available
Melting Point (ºC): Not Available
Boiling Point (ºC): Not Available
Evaporation Rate: Not Available
pH Value: Not Available
Density (g/cm3): 2.2
Refractive Index (n²ºD): Not Available
Viscosity: Not Available
Solubility in Water: Not Available
Solubility in Other: Not Available
Vapor Pressure (mmHg): Not Available
Vapor Density (Air=1): Not Available
SECTION 10. STABILITY AND REACTIVITY
Stability: Stable under normal temperatures and pressures.
Incompatibility: Strong oxidizing agents
Reactivity: Product may react with incompatible materials to release other
hazardous substances.
Conditions to Avoid: Heat, flame, sparks, other ignition sources.
Hazardous
Decomposition Products:
Barium oxides, Carbon oxides, Nitrogen oxides, Sulfur oxides
SECTION 11. TOXICOLOGICAL INFORMATION
RTECS Reference: Not Available
Target Organs: Not Available
Toxicity Data: Not Available
Carcinogenicity: National Toxicology Program (NTP) listed: Not Available
International Agency for Research on Cancer (IARC) listed: Not Available
Potential Symptoms: Not Available
SECTION 12. ECOLOGICAL INFORMATION
Toxicity: Not Available
SECTION 13. DISPOSAL CONSIDERATIONS
Contact a licensed professional waste disposal service. Dispose in a manner consistent with
federal, state and local environmental regulations.
SECTION 14. TRANSPORT INFORMATION
DOT Shipping Name: Barium Compound, N.O.S.
DOT UN Number: UN1564
DOT Hazard Class: Class 6.1
DOT Packing Group: PGII
IMDG Shipping Name: Barium Compound, N.O.S.
IMDG UN Number: UN1564
IMDG Hazard Class: Class 6.1
IMDG Packing Group: PGII
Marine Pollutant: No
IATA: Barium Compound, N.O.S.
IATA UN Number: UN1564
IATA Hazard Class: Class 6.1
IATA Packing Group: PGII
SECTION 15. REGULATORY INFORMATION
United States
Toxic Substance Control Act (TSCA) listed: Yes
Superfund Amendments and Reauthorization Act (SARA 302) listed: No
Superfund Amendments and Reauthorization Act (SARA 311/312) listed: No
Superfund Amendments and Reauthorization Act (SARA 313) listed: No
European Union
European Inventory of Existing Chemical Substances (EINECS): 218-245-9
Canada
Canadian Domestic Substances List (DSL) listed: No
Canadian Non-Domestic Substances List (NDSL) listed: Yes
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 Barium is a member of the alkaline-earth metals. The barium atom has a radius of 222 pm and a Van der Waals radius of 268 pm. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808.
Barium is a member of the alkaline-earth metals. The barium atom has a radius of 222 pm and a Van der Waals radius of 268 pm. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808.  In its elemental form, barium is a soft, silvery-gray metal. Industrial applications for barium include acting as a "getter," or unwanted gas remover, for vacuum tubes, and as an additive to steel and cast iron. Barium is also alloyed with silicon and aluminum in load-bearing alloys. The main commercial source of barium is the mineral barite (BaSO4); it does not occur naturally as a free element . The name barium is derived from the Greek word "barys," meaning heavy.
In its elemental form, barium is a soft, silvery-gray metal. Industrial applications for barium include acting as a "getter," or unwanted gas remover, for vacuum tubes, and as an additive to steel and cast iron. Barium is also alloyed with silicon and aluminum in load-bearing alloys. The main commercial source of barium is the mineral barite (BaSO4); it does not occur naturally as a free element . The name barium is derived from the Greek word "barys," meaning heavy. The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.