See more Chromium products. Chromium (atomic symbol: Cr, atomic number: 24) is a Block D, Group 6, Period 4 element with an atomic weight of 51.9961.  The number of electrons in each of Chromium's shells is 2, 8, 13, 1 and its electron configuration is [Ar] 3d5 4s1. Louis Nicolas Vauquelin first discovered chromium in 1797 and first isolated it the following year. The chromium atom has a radius of 128 pm and a Van der Waals radius of 189 pm. In its elemental form, chromium has a lustrous steel-gray appearance.
The number of electrons in each of Chromium's shells is 2, 8, 13, 1 and its electron configuration is [Ar] 3d5 4s1. Louis Nicolas Vauquelin first discovered chromium in 1797 and first isolated it the following year. The chromium atom has a radius of 128 pm and a Van der Waals radius of 189 pm. In its elemental form, chromium has a lustrous steel-gray appearance.  Chromium is the hardest metallic element in the periodic table and the only element that exhibits antiferromagnetic ordering at room temperature, above which it transforms into a paramagnetic solid. The most common source of chromium is chromite ore (FeCr2O4). Due to its various colorful compounds, Chromium was named after the Greek word 'chroma.' meaning color.
Chromium is the hardest metallic element in the periodic table and the only element that exhibits antiferromagnetic ordering at room temperature, above which it transforms into a paramagnetic solid. The most common source of chromium is chromite ore (FeCr2O4). Due to its various colorful compounds, Chromium was named after the Greek word 'chroma.' meaning color.
Materials
Materials by Form
2D Materials Alloy & Alloy Forms Pure Metals & Metal FormsCeramic FibersFoams: Metallic & Ceramic High Purity Materials Isotopes MXenesOxides Rare Earths Semiconductors Solutions
Chemicals & Salts
All Chemicals & Salts Acetates Aluminides Ammonium Sulfates Antimonides Arsenates Benzoate Bromates Bromides Carbonates Chlorides Chromates Fluorides Hydrides Hydroxides Iodates Iodides Lactates Molybdates Nitrates Oxalates Oxides Perchlorates Phosphates Selenates Selenides Selenites Silicates Stearates Sulfates Sulfides Sulfites Tantalates Tellurates Tellurides Tellurites ThiocyanatesVanadates
Ceramics
Nanomaterials
Organometallics
Materials by Application
Additive Manufacturing & 3D Printing Battery & Supercapacitor Materials Catalysts Dental Materials Electronics Materials Fuel Cell Materials Fusion EnergyGlass Manufacturing Green Technology & Alternative Energy Hydrogen Storage Laser Crystals Life Sciences & Biomaterials Metallurgy Nanotechnology & Nanomaterials Optical Materials Photovoltaic & Solar Energy Plating Pigments & Coatings Research & Development Space Technology Sputtering Targets Thin Film Deposition Water Treatment Weather Modification
Life Science Chemicals
Life Science Products AlcoholsAldehydesAmidesAminesAmino Acids & DerivativesAromaticsArylsAzetidinesBenzimidazolesBenzisoxazolesBenzodioxansBenzofuransBenzothiazolesBenzothiophenesBenzoxazolesCarboxylic AcidsEnzymes & InhibitorsEstersEthersFluorinated Building BlocksFuransHalidesImidazolesImidazolidinesIndazolesIndolesIndolinesIsoquinolinesIsoxazolesKetonesMorpholinesNaphthyridinesNitrilesOrganoboronOrganosiliconOxadiazolesOxazolesPharmaceuticals & IntermediatesPhenolsPhytochemicalsPiperazinesPiperidinesPyrazinesPyrazolesPyridazinesPyridinesPyrimidinesPyrrolesPyrrolidinesPyrrolinesQuinazolinesQuinolinesQuinoxalinesSpiroesSulfonyl ChloridesTetrahydroisoquinolinesTetrahydropyransTetrahydroquinolinesTetrazolesThiadiazolesThiazolesThiazolidinesThiolsThiophenesTriazinesTriazoles
About Us
Locations
Austria Belgium Brazil Canada China & Hong Kong Czech Republic Denmark Finland France Germany Greece Hungary India Indonesia Israel Italy Japan Malaysia Mexico Netherlands Norway Philippines Poland Portugal Russia Singapore South Korea Spain Sweden Switzerland Taiwan Thailand Turkey United Kingdom United States
Industries
Aerospace Agriculture Automotive Chemical Manufacturing Defense Dentistry Electronics Energy Storage & Batteries Fine Art Materials Fuel CellsFusion Energy Glass Investment Grade Metals Jewelry & Fashion Lasers Lighting Medical Devices Museums & Galleries Nuclear Energy Oil & Gas Optics Paper & Pulp Pharmaceuticals & Cosmetics Research & Laboratory Robotics Solar Energy Space Sports Equipment Steel & Alloy Producers Textiles & Fabrics Water Treatment Municipalities
Follow Us
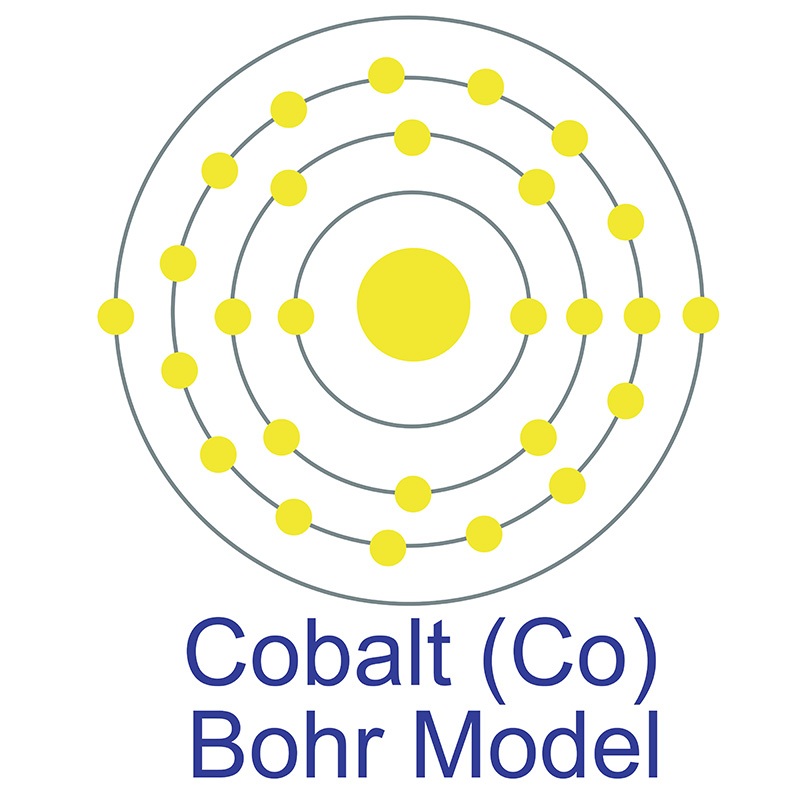 The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar]3d7 4s2. The cobalt atom has a radius of 125 pm and a Van der Waals radius of 192 pm. Cobalt was first discovered by George Brandt in 1732. In its elemental form, cobalt has a lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores.
The number of electrons in each of cobalt's shells is 2, 8, 15, 2 and its electron configuration is [Ar]3d7 4s2. The cobalt atom has a radius of 125 pm and a Van der Waals radius of 192 pm. Cobalt was first discovered by George Brandt in 1732. In its elemental form, cobalt has a lustrous gray appearance. Cobalt is found in cobaltite, erythrite, glaucodot and skutterudite ores.  Cobalt produces brilliant blue pigments which have been used since ancient times to color paint and glass. Cobalt is a ferromagnetic metal and is used primarily in the production of
Cobalt produces brilliant blue pigments which have been used since ancient times to color paint and glass. Cobalt is a ferromagnetic metal and is used primarily in the production of