SECTION 1. IDENTIFICATION
Product Name: Copper Nitrate Solution
Product Number: All applicable American Elements product codes, e.g. CU2-NAT-02-SOL
, CU2-NAT-03-SOL
, CU2-NAT-04-SOL
, CU2-NAT-05-SOL
CAS #: 13778-31-9
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification in accordance with 29 CFR 1910.1200.
Acute toxicity, Inhalation, Category 3
Skin corrosion, Category 1B
Serious eye damage/Irritation, Category 1
Specific Target Organ Toxicity - Single Exposure, Category 3 (respiratory system) Specific Target Organ Toxicity - Repeated Exposure, Category 1 (liver)
Hazard to aquatic life – Acute Hazard, Category 1
Hazard to aquatic life – Chronic Hazard, Category 1
GHS LABEL ELEMENTS Symbol(s)
Signal Word
DANGER
Hazard Statement(s)
May cause respiratory irritation. Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes damage to organs through prolonged or repeated exposure. Very toxic to aquatic life.
Precautionary Statement(s) Prevention
Obtain special instructions before use. Do not breathe vapor or mist. Use only outdoors or in a well-ventilated area. Wear protective gloves/clothing and eye/face protection. Contaminated work clothing should not be allowed out of the workplace. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. Avoid release to the environment.
Response
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF ON SKIN (or hair): Remove/take off immediately all contaminated clothing. Rinse skin with water/shower. Wash contaminated clothing before reuse. If skin irritation or rash occurs: Get medical advice/attention.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER or doctor/physician.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. Call a POISON CENTER or doctor/physician.
Storage
Store in a well-ventilated place. Keep container tightly closed. Store locked up.
Disposal
Dispose in accordance with all applicable regulations.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Water: 7732-18-5
Cupric nitrate: 3251-23-8
Nitric acid: 7697-37-2
Component Related Regulatory Information
This product may be regulated, have exposure limits or other information identified as the following: Copper (7440-50-8), Copper compounds, n.o.s., Copper (inorganic salts), Water Dissociable Nitrate Compounds.
Component Information/Information on Non-Hazardous Components
This product is considered hazardous under 29 CFR 1910.1200 (Hazard Communication).
Emergency Overview
This product is a medium to dark blue liquid with an acrid odor. This product is an oxidizer in is dry form. This product is an irritant and has a corrosive potential. Contact with mists, sprays or liquid product can severely irritate or burn eyes, skin, and other contaminated tissue. Eye contact may cause blindness. Severe inhalation and ingestion overexposures may be fatal.
Potential Health Effects: Eyes
Contact with the eyes will cause irritation, pain, reddening, and may result in blindness depending on the duration.
Potential Health Effects: Skin
This product is moderately irritating to the skin and other contaminated tissue. Depending on the duration of contact, symptoms will include reddening, discomfort, irritation, ulceration, and chemical burns. Prolonged and/or repeated skin contact with this product may cause irritation/dermatitis. Skin absorption is not a significant route of overexposure.
Potential Health Effects: Ingestion
Ingestion of this product can be harmful or fatal. Immediately upon contact, this product will cause irritation and burns of the mouth, throat, esophagus, and other tissues of the digestive system. Overexposure symptoms include: drowsiness, confusion, difficulty swallowing, a burning sensation in the esophagus and stomach, intense thirst, nausea, abdominal pain, vomiting, diarrhea, stomach perforation, bloody stools or urine, convulsions, and collapse. Large quantity ingestion may be fatal.
Potential Health Effects: Inhalation
Inhalation of vapors, mists, or sprays of this product may irritate the nose, throat, and lungs. Symptoms may include: sneezing, coughing and difficulty breathing. Severe overexposures can result in pulmonary edema, pneumonitis, and death.
HMIS Ratings: Health: 3 Fire: 0 Physical Hazard: 0
SECTION 4. FIRST AID MEASURES
Description of Necessary Measures
Eyes
In case of contact, immediately flush eyes with large amounts of water, continuing to flush for 15 minutes. Have contaminated individual "roll" their eyes. Seek immediate medical attention.
Skin
Immediately take off all contaminated clothing. For skin contact, flush with large amounts of water. If irritation persists, get medical attention.
Ingestion
Do not induce vomiting. Call a physician immediately.
Inhalation
Move person to non-contaminated air. Call a physician if symptoms develop or persist.
Notes to Physician
Provide general supportive measures and treat symptomatically.
Most Important Symptoms/Effects
Acute
Eyes
Contact with the eyes will cause irritation, pain, reddening, and may result in blindness depending on the duration.
Skin
This product is moderately irritating to the skin and other contaminated tissue. Depending on the duration of contact, symptoms will include reddening, discomfort, irritation, ulceration, and chemical burns. Prolonged and/or repeated skin contact with this product may cause irritation/dermatitis. Skin absorption is not a significant route of overexposure.
Ingestion
Ingestion of this product can be harmful or fatal. Immediately upon contact, this product will cause irritation and burns of the mouth, throat, esophagus, and other tissues of the digestive system. Overexposure symptoms include: drowsiness, confusion, difficulty swallowing, a burning sensation in the esophagus and stomach, intense thirst, nausea, abdominal pain, vomiting, diarrhea, stomach perforation, bloody stools or urine, convulsions, and collapse. Large quantity ingestion may be fatal.
Inhalation
Inhalation of vapors, mists, or sprays of this product may irritate the nose, throat, and lungs. Symptoms may include: sneezing, coughing and difficulty breathing. Severe overexposures can result in pulmonary edema, pneumonitis, and death.
SECTION 5. FIREFIGHTING MEASURES
General Fire Hazards
This product is an aqueous mixture, which will not burn. If evaporated to dryness, the solid residue may pose a slight fire hazard. This product is an oxidizing agent, which may cause spontaneous ignition of combustible materials.
Hazardous Combustion Products
Decomposition of this product may produce acrid vapors, copper compounds, and oxides of nitrogen.
Extinguishing Media
Use any media suitable for the surrounding fires.
Fire Fighting Equipment/Instructions
Fire fighters should wear full-face, self-contained breathing apparatus and impervious protective clothing. Fire fighters should avoid inhaling any combustion products.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Containment Procedures
Stop the flow of material, if this is without risk. Wear appropriate protective equipment and clothing during clean up. Contain the discharged material and dike the spilled material where possible. Prevent entry into sewers, drains, underground or confined spaces, water intakes and waterways. Avoid contact with combustible materials.
Clean-Up Procedures
Absorb spill with inert material. Shovel material into appropriate container for disposal.
Evacuation Procedures
Isolate area. Keep unnecessary personnel away.
Special Procedures
Follow all Local, State, Federal and Provincial regulations for disposal.
SECTION 7. HANDLING AND STORAGE
Handling Procedures
Do not get this material in your eyes, on your skin, or on your clothing. Avoid breathing vapors or mists of this product. Wash thoroughly after handling. Do not eat, drink or use tobacco products when handling this material. Use this product with adequate ventilation. Launder work clothes frequently. See Section 8 for appropriate protective clothing, equipment and air monitoring procedures.
Open containers slowly, on a stable surface. Containers of this product must be properly labeled. Empty containers may contain residual liquid or vapors. Empty containers should be handled with care.
Storage Procedures
Store containers in a cool, dry location, away from direct sunlight, sources of intense heat, or where freezing is possible. Store away from incompatible materials (see SECTION 10: Stability and Reactivity). Material should be stored in secondary containers, or in a diked area, as appropriate. Keep container tightly closed when not in use. Inspect all incoming containers before storage, to ensure containers are properly labeled and not damaged.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Cupric nitrate (3251-23-8)
ACGIH:0.2 mg/m3 TWA (fume); 1 mg/m3 TWA (dusts and mists, as Cu) (related to Copper)OSHA
Vacated:0.1 mg/m3 TWA (fume, dusts, mists as Cu) (related to Copper)OSHA Final:0.1 mg/m3 TWA (fume); 1 mg/m3 TWA (dusts and mists) (related to Copper) NIOSH:1 mg/m3 TWA (dust and mist) (related to Copper)
Nitric acid (7697-37-2)
ACGIH:2 ppm TWA
4 ppm STELOSHA
Vacated:2 ppm TWA; 5 mg/m3 TWA
4 ppm STEL; 10 mg/m3 STELOSHA Final:2 ppm TWA; 5 mg/m3 TWANIOSH:2 ppm TWA; 5 mg/m3 TWA
4 ppm STEL; 10 mg/m3 STEL
Engineering Controls
Provide adequate local exhaust ventilation to maintain worker exposure below exposure limits.
PERSONAL PROTECTIVE EQUIPMENT Personal Protective Equipment: Eyes/Face
Wear safety glasses; chemical goggles (if splashing is possible).
Personal Protective Equipment: Skin
Use impervious gloves. Use of an impervious apron is recommended.
Personal Protective Equipment: Respiratory
Respiratory protection; not normally required for ambient air concentrations not exceeding the Occupational Exposure Limit. When respiratory protection is required, wear a NIOSH/MSHA approved self-contained breathing apparatus with full-face piece operated in a positive-pressure mode.
Personal Protective Equipment: General
Eyewash fountains and emergency showers are required.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance: Medium to dark blue
Odor: Acrid odor
Physical State: liquid
pH: 0-0.6
Evaporation Rate: Not Established
Odor Threshold: Not Applicable
Vapor Pressure: Not Applicable
Vapor Density: Not Applicable
Freezing Point: Not Established
Viscosity: Not Established
Relative Density:Not Applicable
Partition coefficient: n- octanol/water: Not Established
Initial Boiling Point and boiling range: Not Established
Melting Point: Not Established
Solubility (H2O): Soluble
Specific Gravity: 1.44-1.65
Flash Point: Not Flammable
Upper Flammable Limit (UFL): Not Applicable
Lower Flammable Limit (LFL): Not Applicable
Auto Ignition: Not Available
Flammability Classification: Not Applicable
Rate of Burning: Not Applicable
SECTION 10. STABILITY AND REACTIVITY
Chemical Stability
Stable under normal conditions.
Chemical Stability: Conditions to Avoid
Avoid exposure to extreme temperatures, contact with incompatible chemicals, and all contact with combustible materials.
Incompatibility
Strong bases, active metals (e.g., sodium, potassium), cyanide compounds, flammable and combustible materials, strong reducing agents, finely powdered metals.
Hazardous Decomposition
Copper compounds and nitrogen oxides.
Hazardous Polymerization
Will not occur.
SECTION 11. TOXICOLOGICAL INFORMATION
General Product Information
This product is an irritant. Depending on the duration, contact can mildly to severely irritate the eyes, skin, mucous membranes, and any other exposed tissue. Inhalation may cause irritation of the respiratory system with coughing and difficulty breathing. Skin contact may cause blisters and scars. Eye contact may cause blindness. Severe inhalation and ingestion overexposures may be fatal.
B: Component Analysis - LD50/LC50
Cupric nitrate (3251-23-8)
Oral LD50 Rat: 794 mg/kg
100 mg/mm3 IDLH (dust, fume and mist) (related to Copper)
Nitric acid (7697-37-2)
Inhalation LC50 Rat: 7 mg/L/4H
25 ppm IDLH
Carcinogenicity
A: General Product Information
No carcinogenicity data available for this product.
B: Component Carcinogenicity
None of this product's components are listed by ACGIH, IARC, OSHA, NIOSH, or NTP.
Other Toxicological Information
Target Organs: Skin, eyes, respiratory system
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
A: General Product Information
In high concentrations, this product may be dangerous to aquatic life and fouling to shorelines.
B: Component Analysis - Ecotoxicity - Aquatic Toxicity
Test & Species Conditions
96 Hr LC50 fathead minnow 23 µg/L
96 Hr LC50 rainbow trout 13.8 µg/L
96 Hr LC50 bluegill 236 µg/L related to Copper
72 Hr EC50 freshwater algae (Scenedesmus subspicatus) 120 µg/L related to Copper
96 Hr LC50 water flea 10 µg/L
96 Hr LC50 water flea 200 µg/L related to Copper
Environmental Fate
Due to the low pH associated with this product, plants contaminated with this product may be adversely affected or destroyed. Animals contaminated with this solution may be severely injured or killed.
SECTION 13. DISPOSAL CONSIDERATIONS
US EPA Waste Number & Descriptions
A: General Product Information
Wastes must be tested using methods described in 40 CFR Part 261 to determine if it meets applicable definitions of hazardous wastes. As packaged this product is a D002 corrosive waste [40 CFR 261.21(a)(4)]; applicable to wastes consisting only of this product.
B: Component Waste Numbers
No EPA Waste Numbers are applicable for this product's components.
Disposal Instructions
Dispose of waste material according to Local, State, Federal, and Provincial Environmental Regulations.
SECTION 14. TRANSPORT INFORMATION
US DOT Information
Shipping Name: Corrosive liquid, acidic, inorganic, n.o.s. (Copper nitrate, nitric acid)
UN/NA #: UN3264 Hazard Class: 8 Packing Group: II
Required Label(s): Corrosive
Response Guide #: 154
Canada Transportation of Dangerous Goods Information
Shipping Name: Corrosive liquid, acidic, inorganic, n.o.s. (Copper nitrate, nitric acid)
UN/NA #: UN3264 Hazard Class: 8 Packing Group: II
Required Label(s): Corrosive
International Maritime Dangerous Goods Information
Shipping Name: Corrosive liquid, acidic, inorganic, n.o.s. (Copper nitrate, nitric acid)
UN/NA #: UN3264 Hazard Class: 8 Packing Group: II
Required Label(s): Corrosive
EMS: S-B, F-A
SECTION 15. REGULATORY INFORMATION
US Federal Regulations
A: General Product Information
Components of this product have been checked against the non-confidential TSCA inventory by CAS Registry Number. Components not identified on this non-confidential inventory are exempt from listing (i.e. as polymers) or are listed on the confidential inventory as declared by the supplier.
B: Component Analysis
This material contains one or more of the following chemicals required to be identified under SARA Section 302 (40 CFR 355 Appendix A), SARA Section 313 (40 CFR 372.65) and/or CERCLA (40 CFR 302.4).
SARA 313:1.0 % de minimis concentration (related to Copper) 1.0 % de minimis concentration (Chemical Category N511) (related to Water Dissociable Nitrate Compounds)CERCLA:100 lb final RQ; 45.4 kg final RQ
Nitric acid (7697-37-2)
C: Federal Insecticide, Fungicide, and Rodenticide Act
This material contains the following chemicals present on either the Listing of Pesticide Chemicals (40 CFR 180)
or Pesticides Classified for Restricted Use as listed by FIFRA:
Cupric nitrate (3251-23-8)
FIFRA Section number 180.538 (related to Copper)
D: Component Marine Pollutants
This material contains one or more of the following chemicals required by US DOT to be identified as marine pollutants.
Component CAS DOT
Cupric nitrate (related to Copper, metal powder) 3251-23-8 regulated severe marine pollutant
SARA 311/312: Acute Health Yes Chronic Health No Fire No Pressure No Reactive Yes
State Regulations
A: General Product Information
Other state regulations may apply. Check individual state requirements.
B: Component Analysis - State
The following components appear on one or more of the following state hazardous substances lists:
Component CAS CA MA MN NJ PA RI
Cupric nitrate (¹related to Copper) 3251-23-8 Yes Yes Yes Yes Yes Yes
Nitric acid 7697-37-2 Yes Yes Yes Yes Yes Yes
Component Analysis - WHMIS IDL
The following components are identified under the Canadian Hazardous Products Act Ingredient Disclosure List:
Component CAS Minimum Concentration
Cupric nitrate 3251-23-8 1 % (English Item 436, French Item 1203)
Additional Regulatory Information
A: General Product Information
No additional information available.
B: Component Analysis - Inventory
Component CAS TSCA DSL NDSL EINECS AUST MITI PHIL KOREA ELINCS CHINA
Water 7732-18-5 Yes Yes No Yes Yes Yes No Yes No Yes
Cupric nitrate 3251-23-8 Yes Yes No Yes Yes Yes No Yes No Yes
Nitric acid 7697-37-2 Yes Yes No Yes Yes Yes No Yes No Yes
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 materials. American Elements can prepare dissolved homogeneous solutions at customer specified concentrations or to the maximum stoichiometric concentration. Packaging is available in 55 gallon drums, smaller units and larger liquid totes. American Elements maintains
materials. American Elements can prepare dissolved homogeneous solutions at customer specified concentrations or to the maximum stoichiometric concentration. Packaging is available in 55 gallon drums, smaller units and larger liquid totes. American Elements maintains 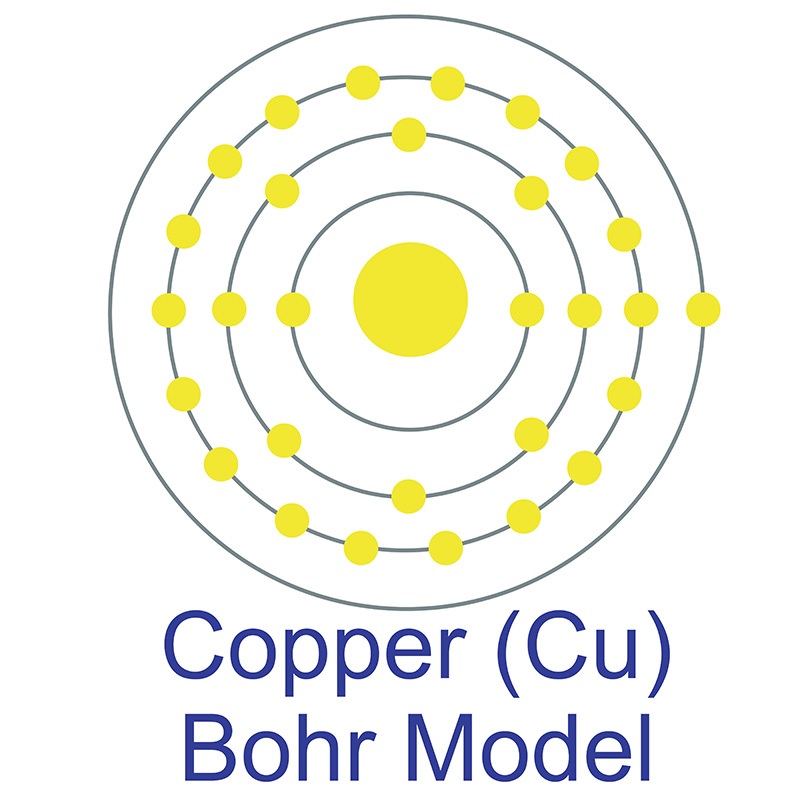 Copper (atomic symbol: Cu, atomic number: 29) is a Block D, Group 11, Period 4 element with an atomic weight of 63.546. The number of electrons in each of copper's shells is 2, 8, 18, 1 and its electron configuration is [Ar]3d10 4s1. The copper atom has a radius of 128 pm and a Van der Waals radius of 186 pm. Copper was first discovered by Early Man prior to 9000 BC. In its elemental form, copper has a reddish-orange metallic and lustrous appearance. Of all pure
Copper (atomic symbol: Cu, atomic number: 29) is a Block D, Group 11, Period 4 element with an atomic weight of 63.546. The number of electrons in each of copper's shells is 2, 8, 18, 1 and its electron configuration is [Ar]3d10 4s1. The copper atom has a radius of 128 pm and a Van der Waals radius of 186 pm. Copper was first discovered by Early Man prior to 9000 BC. In its elemental form, copper has a reddish-orange metallic and lustrous appearance. Of all pure  has a higher electrical conductivity. The origin of the word copper comes from the Latin word 'cuprium' which translates as "metal of Cyprus," as the Mediterranean island of Cyprus was known as an ancient source of mined copper..
has a higher electrical conductivity. The origin of the word copper comes from the Latin word 'cuprium' which translates as "metal of Cyprus," as the Mediterranean island of Cyprus was known as an ancient source of mined copper..