SECTION 1. IDENTIFICATION
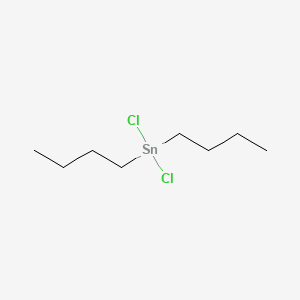
Product Name: Dibutyltin Dichloride
Product Number: All applicable American Elements product codes, e.g. DBSN-CL-01-CC
CAS #: 683-18-1
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
GHS Classification in accordance with 29 CFR 1910 (OSHA HCS)
Acute toxicity, Oral (Category 3), H301
Acute toxicity, Inhalation (Category 2), H330
Acute toxicity, Dermal (Category 4), H312
Skin corrosion (Category 1B), H314
Serious eye damage (Category 1), H318
Skin sensitization (Category 1), H317
Germ cell mutagenicity (Category 2), H341
Reproductive toxicity (Category 1B), H360
Specific target organ toxicity - single exposure, Oral (Category 1), thymus, H370
Specific target organ toxicity - repeated exposure, Oral (Category 1), thymus, H372
Short-term (acute) aquatic hazard (Category 1), H400
Long-term (chronic) aquatic hazard (Category 1), H410
Signal Word Danger
Hazard statement(s)
H301 Toxic if swallowed.
H312 Harmful in contact with skin.
H314 Causes severe skin burns and eye damage.
H317 May cause an allergic skin reaction.
H330 Fatal if inhaled.
H341 Suspected of causing genetic defects.
H360 May damage fertility or the unborn child.
H370 Causes damage to organs (thymus) if swallowed.
H372 Causes damage to organs (thymus) through prolonged or
repeated exposure if swallowed.
H410 Very toxic to aquatic life with long lasting effects.
Precautionary statement(s)
P201 Obtain special instructions before use.
P202 Do not handle until all safety precautions have been read and
understood.
P260 Do not breathe dust/ fume/ gas/ mist/ vapors/ spray.
P264 Wash skin thoroughly after handling.
P270 Do not eat, drink or smoke when using this product.
P271 Use only outdoors or in a well-ventilated area.
P272 Contaminated work clothing must not be allowed out of the
workplace.
P273 Avoid release to the environment.
P280 Wear protective gloves/ protective clothing/ eye protection/ face
protection.
P284 Wear respiratory protection.
P301 + P310 + P330 IF SWALLOWED: Immediately call a POISON CENTER/ doctor.
Rinse mouth.
P301 + P330 + P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P303 + P361 + P353 IF ON SKIN (or hair): Take off immediately all contaminated
clothing. Rinse skin with water/ shower.
P304 + P340 + P310 IF INHALED: Remove person to fresh air and keep comfortable
for breathing. Immediately call a POISON CENTER/ doctor.
P305 + P351 + P338 +
P310
IF IN EYES: Rinse cautiously with water for several minutes.
Remove contact lenses, if present and easy to do. Continue
rinsing. Immediately call a POISON CENTER/ doctor.
P307 + P311 IF exposed: Call a POISON CENTER or doctor/ physician.
P333 + P313 If skin irritation or rash occurs: Get medical advice/ attention.
P363 Wash contaminated clothing before reuse.
P391 Collect spillage.
P403 + P233 Store in a well-ventilated place. Keep container tightly closed.
P405 Store locked up.
P501 Dispose of contents/ container to an approved waste disposal plant.
Hazards not otherwise classified (HNOC) or not covered by GHS - none
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : Dibutyldichlorotin
Formula : C8H18Cl2Sn
Molecular weight : 303.84 g/mol
CAS-No. : 683-18-1
EC-No. : 211-670-0
Index-No. : 050-022-00-X
SECTION 4. FIRST AID MEASURES
Description of first-aid measures
General advice
First aiders need to protect themselves. Show this material safety data sheet to the doctor
in attendance.
If inhaled
After inhalation: fresh air. Immediately call in physician. If breathing stops: immediately
apply artificial respiration, if necessary also oxygen.
In case of skin contact
In case of skin contact: Take off immediately all contaminated clothing. Rinse skin with
water/ shower. Call a physician immediately.
In case of eye contact
After eye contact: rinse out with plenty of water. Immediately call in ophthalmologist.
Remove contact lenses.
If swallowed
If swallowed: give water to drink (two glasses at most). Seek medical advice immediately.
In exceptional cases only, if medical care is not available within one hour, induce vomiting
(only in persons who are wide awake and fully conscious), administer activated charcoal
(20 - 40 g in a 10% slurry) and consult a doctor as quickly as possible. Do not attempt to
neutralise.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section
2.2) and/or in section 11
Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Water Foam Carbon dioxide (CO2) Dry powder
Unsuitable extinguishing media
For this substance/mixture no limitations of extinguishing agents are given.
Special hazards arising from the substance or mixture
Carbon oxides
Hydrogen chloride gas
Tin/tin oxides
Combustible.
Vapors are heavier than air and may spread along floors.
Forms explosive mixtures with air on intense heating.
Development of hazardous combustion gases or vapours possible in the event of fire.
Advice for firefighters
Stay in danger area only with self-contained breathing apparatus. Prevent skin contact by
keeping a safe distance or by wearing suitable protective clothing.
Further information
Suppress (knock down) gases/vapors/mists with a water spray jet. Prevent fire
extinguishing water from contaminating surface water or the ground water system.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Advice for non-emergency personnel: Avoid generation and inhalation of dusts in all
circumstances. Avoid substance contact. Ensure adequate ventilation. Evacuate the
danger area, observe emergency procedures, consult an expert.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Cover drains. Collect, bind, and pump off spills. Observe possible material restrictions
(see sections 7 and 10). Take up carefully. Dispose of properly. Clean up affected area.
Avoid generation of dusts.
Reference to other sections
For disposal see section 13.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling
Advice on safe handling
Work under hood. Do not inhale substance/mixture.
Hygiene measures
Immediately change contaminated clothing. Apply preventive skin protection. Wash hands
and face after working with substance.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Storage conditions
Tightly closed. Dry. Keep in a well-ventilated place. Keep locked up or in an area accessible
only to qualified or authorized persons.
Moisture sensitive.
Storage class
Storage class (TRGS 510): 6.1A: Combustible, acute toxic Cat. 1 and 2 / very toxic
hazardous materials
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Exposure controls
Appropriate engineering controls
Immediately change contaminated clothing. Apply preventive skin protection. Wash
hands and face after working with substance.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate
government standards such as NIOSH (US) or EN 166(EU). Tightly fitting safety
goggles
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove
removal technique (without touching glove's outer surface) to avoid skin contact
with this product. Dispose of contaminated gloves after use in accordance with
applicable laws and good laboratory practices. Wash and dry hands.
Body Protection
protective clothing
Respiratory protection
required when dusts are generated.
Our recommendations on filtering respiratory protection are based on the following
standards: DIN EN 143, DIN 14387 and other accompanying standards relating to
the used respiratory protection system.
Control of environmental exposure
Do not let product enter drains.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance Form: Solidified mass or fragments
Color: colorless
Odor stinging
Odor Threshold No data available
pH No data available
Melting point/freezing point
Melting point/range: 37 - 40 °C (99 - 104 °F) - lit.
Initial boiling point and boiling range
135 °C 275 °F at 13 hPa - lit.
Flash point 113 °C (235 °F) - closed cup
Evaporation rate No data available
Flammability (solid, gas) No data available
Upper/lower flammability or explosive limits No data available
Vapor pressure 0.0016 hPa at 25 °C (77 °F) - OECD Test Guideline 104
Vapor density No data available
Density 1.4 g/cm3 at 20 °C (68 °F) - OECD Test Guideline 109
Relative density No data available
Water solubility 0.32 g/l at 20 °C (68 °F)soluble
Partition coefficient: n-octanol/water
log Pow: 1.56 - (experimental) - Bioaccumulation is not
expected., (Lit.)
Autoignition temperature No data available
Decomposition temperature > 230 °C (> 446 °F) -
Viscosity No data available
Explosive properties No data available
Oxidizing properties none
SECTION 10. STABILITY AND REACTIVITY
Reactivity
Forms explosive mixtures with air on intense heating.
A range from approx. 15 Kelvin below the flash point is to be rated as critical.
The following applies in general to flammable organic substances and mixtures: in
correspondingly fine distribution, when whirled up a dust explosion potential may generally
be assumed.
Chemical stability
The product is chemically stable under standard ambient conditions (room temperature) .
Possibility of hazardous reactions
Violent reactions possible with:
Oxidizing agents
Conditions to avoid
Heat.
Strong heating.
Incompatible materials
No data available
Hazardous decomposition products
In the event of fire: see section 5
SECTION 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
LD50 Oral - Rat - male and female - 219 mg/kg
Remarks: (ECHA)
LC50 Inhalation - Rat - male and female - 4 h - 0.059 mg/l - dust/mist
Remarks: (ECHA)
Inhalation: Corrosive to respiratory system.
Dermal: No data available
No data available
Skin corrosion/irritation
Skin - Rabbit
Result: Corrosive - 4 h
(Draize Test)
Remarks: (ECHA)
Classified according to Regulation (EU) 1272/2008, Annex VI (Table 3.1/3.2)
Serious eye damage/eye irritation
Eyes - Rabbit
Result: Causes serious eye damage.
(Draize Test)
Remarks: (ECHA)
Causes serious eye damage.
Respiratory or skin sensitization
Maximization Test - Guinea pig
Result: positive
(OECD Test Guideline 406)
Remarks: (in analogy to similar products)
Germ cell mutagenicity
Suspected of causing genetic defects.
Test Type: Mutagenicity (mammal cell test): chromosome aberration.
Test system: Human lymphocytes
Metabolic activation: with and without metabolic activation
Method: OECD Test Guideline 473
Result: positive
Test Type: Ames test
Test system: Salmonella typhimurium
Metabolic activation: with and without metabolic activation
Method: OECD Test Guideline 471
Result: negative
Test Type: In vitro mammalian cell gene mutation test
Test system: Chinese hamster lung cells
Metabolic activation: with and without metabolic activation
Method: OECD Test Guideline 476
Result: negative
Test Type: Micronucleus test
Species: Mouse
Cell type: Red blood cells (erythrocytes)
Application Route: Oral
Method: OECD Test Guideline 474
Result: positive
Carcinogenicity
IARC: No ingredient of this product present at levels greater than or equal to 0.1% is
identified as probable, possible or confirmed human carcinogen by IARC.
NTP: No ingredient of this product present at levels greater than or equal to 0.1% is
identified as a known or anticipated carcinogen by NTP.
OSHA: No component of this product present at levels greater than or equal to 0.1% is
on OSHA’s list of regulated carcinogens.
Reproductive toxicity
May damage the unborn child.
May damage fertility.
Specific target organ toxicity - single exposure
Oral - Causes damage to organs. - thymus
Specific target organ toxicity - repeated exposure
Oral - Causes damage to organs through prolonged or repeated exposure. - thymus
Remarks: Classified according to Regulation (EU) 1272/2008, Annex VI (Table 3.1/3.2)
Aspiration hazard
No data available
Additional Information
Repeated dose toxicity - Rat - male and female - Oral - NOAEL (No observed adverse effect
level) - 0.3 - 0.4 mg/kg
RTECS: WH7100000
Material is extremely destructive to tissue of the mucous membranes and upper respiratory
tract, eyes, and skin., Cough, Shortness of breath, Headache, Nausea
To the best of our knowledge, the chemical, physical, and toxicological properties have not
been thoroughly investigated.
After absorption:
Damage to:
Liver
The following applies to organic tin compounds in general: systemic effect: CNS disorders
(spasms, narcosis, respiratory paralysis).
Other dangerous properties can not be excluded.
This substance should be handled with particular care.
SECTION 12. ECOLOGICAL INFORMATION
Toxicity
Toxicity to fish semi-static test LC50 - Danio rerio (zebra fish) - > 4 mg/l - 96 h
(OECD Test Guideline 203)
Toxicity to daphnia
and other aquatic
invertebrates
static test EC50 - Daphnia magna (Water flea) - 0.843 mg/l - 48 h
(OECD Test Guideline 202)
Toxicity to algae static test ErC50 - Desmodesmus subspicatus (green algae) - 8 mg/l
- 72 h
(OECD Test Guideline 201)
Persistence and degradability
Biodegradability aerobic - Exposure time 28 d
Result: ca.6 % - Not readily biodegradable.
(OECD Test Guideline 301B)
Bioaccumulative potential
Bioaccumulation Poecilia reticulata (guppy) - 4 Weeks
- 394 µg/l(Dibutyltin chloride)
Bioconcentration factor (BCF): 15
Mobility in soil
No data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not
conducted
Endocrine disrupting properties
No data available
Other adverse effects
Discharge into the environment must be avoided.
SECTION 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Waste material must be disposed of in accordance with the national and local regulations.
Leave chemicals in original containers. No mixing with other waste. Handle uncleaned
containers like the product itself. See www.retrologistik.com for processes regarding the
return of chemicals and containers, or contact us there if you have further questions.
SECTION 14. TRANSPORT INFORMATION
DOT (US)
UN number: 3146 Class: 6.1 Packing group: II
Proper shipping name: Organotin compounds, solid, n.o.s.
Reportable Quantity (RQ):
1) Marine pollutant: yesPoison Inhalation Hazard: No
IMDG
UN number: 3146 Class: 6.1 Packing group: II EMS-No: F-A, S-A
Proper shipping name: ORGANOTIN COMPOUND, SOLID, N.O.S. (Dibutyltin chloride)
Marine pollutant : yes
Marine pollutant : yes
IATA
UN number: 3146 Class: 6.1 Packing group: II
Proper shipping name: Organotin compound, solid, n.o.s. (Dibutyltin chloride)
SECTION 15. REGULATORY INFORMATION
SARA 302 Components
This material does not contain any components with a section 302 EHS TPQ.
SARA 313 Components
This material does not contain any chemical components with known CAS numbers that
exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section
313.
SARA 311/312 Hazards
Acute Health Hazard, Chronic Health Hazard
Massachusetts Right To Know Components
No components are subject to the Massachusetts Right to Know Act.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 In its elemental form, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine. It has the third highest electronegativity and the highest electron affinity of all elements, making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808.
In its elemental form, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine. It has the third highest electronegativity and the highest electron affinity of all elements, making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808. See more Tin products.
See more Tin products. Tin has nine stable isotopes and 18 unstable isotopes. Under 3.72 degrees Kelvin, Tin becomes a superconductor. Applications for tin include
Tin has nine stable isotopes and 18 unstable isotopes. Under 3.72 degrees Kelvin, Tin becomes a superconductor. Applications for tin include