See more Boron products.  Boron (atomic symbol: B, atomic number: 5) is a Block P, Group 13, Period 2 element with an atomic weight of 10.81. The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90 pm and a Van der Waals radius of 192 pm. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808 and was first isolated by Humphry Davy later that year. Boron is classified as a metalloid is not found naturally on earth.
Boron (atomic symbol: B, atomic number: 5) is a Block P, Group 13, Period 2 element with an atomic weight of 10.81. The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90 pm and a Van der Waals radius of 192 pm. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808 and was first isolated by Humphry Davy later that year. Boron is classified as a metalloid is not found naturally on earth.  Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable compounds featuring triple bonds. Boron has an energy band gap of 1.50 to 1.56 eV, which is higher than that of either silicon or germanium. The name Boron originates from a combination of carbon and the Arabic word buraqu meaning borax.
Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable compounds featuring triple bonds. Boron has an energy band gap of 1.50 to 1.56 eV, which is higher than that of either silicon or germanium. The name Boron originates from a combination of carbon and the Arabic word buraqu meaning borax.
Materials
Materials by Form
2D Materials Alloy & Alloy Forms Pure Metals & Metal FormsCeramic FibersFoams: Metallic & Ceramic High Purity Materials Isotopes MXenesOxides Rare Earths Semiconductors Solutions
Chemicals & Salts
All Chemicals & Salts Acetates Aluminides Ammonium Sulfates Antimonides Arsenates Benzoate Bromates Bromides Carbonates Chlorides Chromates Fluorides Hydrides Hydroxides Iodates Iodides Lactates Molybdates Nitrates Oxalates Oxides Perchlorates Phosphates Selenates Selenides Selenites Silicates Stearates Sulfates Sulfides Sulfites Tantalates Tellurates Tellurides Tellurites ThiocyanatesVanadates
Ceramics
Nanomaterials
Organometallics
Materials by Application
Additive Manufacturing & 3D Printing Battery & Supercapacitor Materials Catalysts Dental Materials Electronics Materials Fuel Cell Materials Fusion EnergyGlass Manufacturing Green Technology & Alternative Energy Hydrogen Storage Laser Crystals Life Sciences & Biomaterials Metallurgy Nanotechnology & Nanomaterials Optical Materials Photovoltaic & Solar Energy Plating Pigments & Coatings Research & Development Space Technology Sputtering Targets Thin Film Deposition Water Treatment Weather Modification
Life Science Chemicals
Life Science Products AlcoholsAldehydesAmidesAminesAmino Acids & DerivativesAromaticsArylsAzetidinesBenzimidazolesBenzisoxazolesBenzodioxansBenzofuransBenzothiazolesBenzothiophenesBenzoxazolesCarboxylic AcidsEnzymes & InhibitorsEstersEthersFluorinated Building BlocksFuransHalidesImidazolesImidazolidinesIndazolesIndolesIndolinesIsoquinolinesIsoxazolesKetonesMorpholinesNaphthyridinesNitrilesOrganoboronOrganosiliconOxadiazolesOxazolesPharmaceuticals & IntermediatesPhenolsPhytochemicalsPiperazinesPiperidinesPyrazinesPyrazolesPyridazinesPyridinesPyrimidinesPyrrolesPyrrolidinesPyrrolinesQuinazolinesQuinolinesQuinoxalinesSpiroesSulfonyl ChloridesTetrahydroisoquinolinesTetrahydropyransTetrahydroquinolinesTetrazolesThiadiazolesThiazolesThiazolidinesThiolsThiophenesTriazinesTriazoles
About Us
Locations
Austria Belgium Brazil Canada China & Hong Kong Czech Republic Denmark Finland France Germany Greece Hungary India Indonesia Israel Italy Japan Malaysia Mexico Netherlands Norway Philippines Poland Portugal Russia Singapore South Korea Spain Sweden Switzerland Taiwan Thailand Turkey United Kingdom United States
Industries
Aerospace Agriculture Automotive Chemical Manufacturing Defense Dentistry Electronics Energy Storage & Batteries Fine Art Materials Fuel CellsFusion Energy Glass Investment Grade Metals Jewelry & Fashion Lasers Lighting Medical Devices Museums & Galleries Nuclear Energy Oil & Gas Optics Paper & Pulp Pharmaceuticals & Cosmetics Research & Laboratory Robotics Solar Energy Space Sports Equipment Steel & Alloy Producers Textiles & Fabrics Water Treatment Municipalities
Follow Us
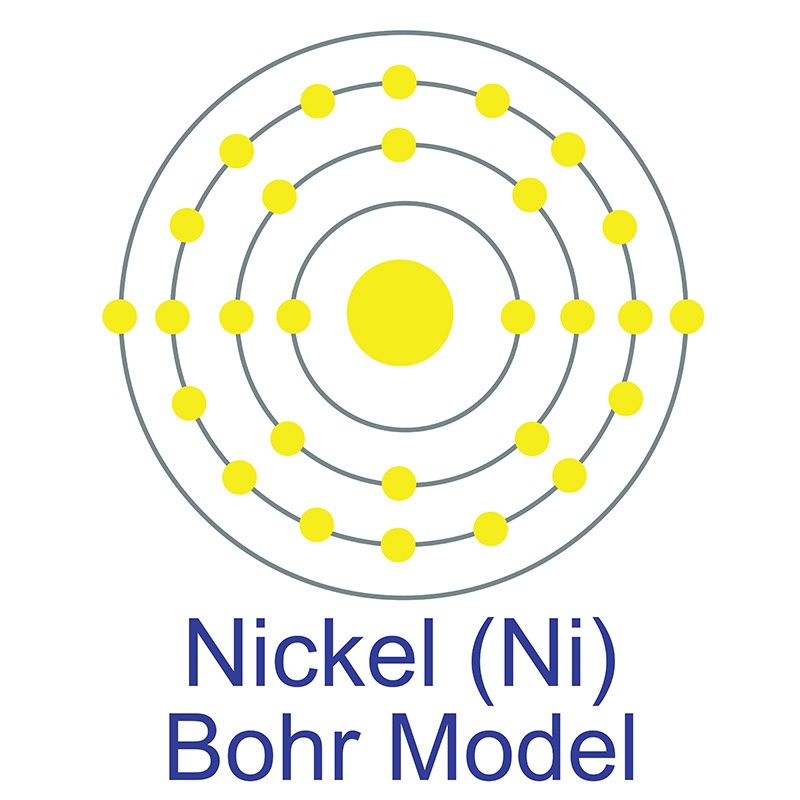 The number of electrons in each of nickel's shells is [2, 8, 16, 2] and its electron configuration is [Ar]3d8 4s2. Nickel was first discovered by Alex Constedt in 1751. The nickel atom has a radius of 124 pm and a Van der Waals radius of 184 pm. In its elemental form, nickel has a lustrous metallic silver appearance. Nickel is a hard and ductile
The number of electrons in each of nickel's shells is [2, 8, 16, 2] and its electron configuration is [Ar]3d8 4s2. Nickel was first discovered by Alex Constedt in 1751. The nickel atom has a radius of 124 pm and a Van der Waals radius of 184 pm. In its elemental form, nickel has a lustrous metallic silver appearance. Nickel is a hard and ductile  It is one of four elements that are ferromagnetic and is used in the production of various type of magnets for commercial use. Nickel is sometimes found free in nature but is more commonly found in ores. The bulk of mined nickel comes from laterite and magmatic sulfide ores. The name originates from the German word kupfernickel, which means "false
It is one of four elements that are ferromagnetic and is used in the production of various type of magnets for commercial use. Nickel is sometimes found free in nature but is more commonly found in ores. The bulk of mined nickel comes from laterite and magmatic sulfide ores. The name originates from the German word kupfernickel, which means "false