SECTION 1. IDENTIFICATION
Product Name: Hafnium Oxynitrate Dihydrate
Product Number: All applicable American Elements product codes, e.g. HF-ONAT-02-C.2HYD
, HF-ONAT-03-C.2HYD
, HF-ONAT-04-C.2HYD
, HF-ONAT-05-C.2HYD
CAS #: 15060-56-7
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification
This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200)
Acute toxicity - Oral Category 4
Skin corrosion/irritation Category 1B
Serious eye damage/eye irritation Category 1
Oxidizing solids Category 2
Label elements
Emergency Overview
Signal Word:
Danger
Hazard statements:
H272: May intensify fire; oxidizer
Causes severe skin burns and eye damage
H314: Causes serious eye damage
H302: Harmful if swallowed
Appearance: Powder
Physical state: Powder
Odor: Pungent, Slight nitric
Precautionary Statements - Prevention
P280: Wear protective gloves/protective clothing/eye protection/face protection
P260: Do not breathe dust/gas/mist
P270: Do not eat, drink or smoke when using this product
P264: Wash hands thoroughly after handling
P210: Keep away from heat/sparks/open flames/hot surfaces. - No smoking
P220: Store away from flammable substances, reducing agents, metal powders, and organic materials.
Precautionary Statements - Response
P301+330+331+P310: IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. Immediately call a POISON CENTER or doctor/physician
P303+P335+P361+P353: IF ON SKIN (or hair): Brush off loose particles from skin, Remove/Take off immediately all contaminated clothing, Rinse skin with
water/shower
P304+340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
P363: Wash contaminated clothing before reuse
P370+378: In case of fire: Use media appropriate for class of fire for extinction.
Precautionary Statements - Storage
P402: Store in a dry place
Precautionary Statements - Disposal
P501: Dispose of contents/container to an approved waste disposal plant
Hazards not otherwise classified (HNOC)
N/A
Other Information
N/A
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Synonyms Hafnium Nitrate Oxide, Hafnium Nitrate Hydroxide, Hafnium Binitrate Dihydroxide, Hafnium
Nitrate (Product #416).
Chemical Name CAS No. Weight-%
Hafnium Dinitrate Oxide 15060-56-7 >99%
SECTION 4. FIRST AID MEASURES
First aid measures
Eye contact Flush with water for 15 minutes. See a physician.
Skin Contact Brush off loose particles from skin. Remove/Take off immediately all contaminated clothing.
Rinse skin with water/shower.
Inhalation IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a physician or poison control center immediately.
Ingestion Do NOT induce vomiting. Have patient drink large quantities of water if able. Call Physician
immediately for further instructions.
Most important symptoms and effects, both acute and delayed
Symptoms May cause acute gastrointestinal effects if swallowed. Contact with moist skin may cause
skin burns. May cause breathing difficulties if inhaled.
Indication of any immediate medical attention and special treatment needed
Note to physicians Treat symptomatically.
SECTION 5. FIREFIGHTING MEASURES
Suitable extinguishing media
Material is a strong oxidizer but is itself not flammable. Select extinguishing media appropriate for class of fire.
Unsuitable extinguishing media: None.
Specific hazards arising from the chemical
This is a strong oxidizer and will react vigorously or explosively with many materials including organic materials, such as wood and
paper, and flammable metals.
Hazardous combustion products: Nitrogen oxide gases may cause respiratory and/or eye irritation.
Explosion data
Sensitivity to Mechanical Impact None.
Sensitivity to Static Discharge None.
Protective equipment and precautions for firefighters
As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH approved (or equivalent) respirator and
full protective gear.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Personal precautions Use personal protective equipment as required.
For emergency responders Use personal protective equipment as required. Follow Emergency Response Guidebook,
Guide No. 140.
Environmental precautions
Environmental precautions Collect spillage to prevent release to the environment.
Methods and material for containment and cleaning up
Methods for containment Prevent further leakage or spillage if safe to do so.
Methods for cleaning up Sweep or shovel material into dry containers. Avoid creating uncontrolled dust. Sweep or
shovel material into dry containers. Avoid creating uncontrolled dust. Wash the spill
location thoroughly with water. Respiratory protection may be needed. Skin and eye
protection should be used during cleanup.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling
Advice on safe handling Protect from moisture: Reacts with water. Ensure adequate ventilation, especially in
confined areas.
Conditions for safe storage, including any incompatibilities Conditions Keep away from heat, sparks, flame and other sources of ignition (i.e., pilot lights, electric
motors and static electricity). Store away from flammable substances, reducing agents,
metal powders, and organic materials.
Incompatible materials Water, alcohols, phenols, and amines. Rubber, coatings, and some plastics. Flammable
substances, reducing agents, metal powders, and organic materials. Reacts with metals to
produce heat and corrosive gases.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Chemical Name ACGIH TLV OSHA PEL
Hafnium Dinitrate Oxide
15060-56-7
- - Appropriate engineering controls
Engineering Controls Avoid generation of uncontrolled particles.
Individual protection measures, such as personal protective equipment
Eye/face protection If a risk of eye injury or irritation is present, appropriate eye protection is recommended; for
example, tight-fitting goggles, foam-lined safety glasses, face shield, or other protective
equipment that shields the eyes.
Skin and body protection Wear impervious protective clothing, including boots, gloves, lab coat, apron or coveralls,
as appropriate, to prevent skin contact.
Respiratory protection When particulates/fumes/gases are generated and if exposure limits are exceeded or
irritation is experienced, proper approved respiratory protection should be worn.
Positive-pressure supplied air respirators may be required for high airborne contaminat
concentrations. Respiratory protection must be provided in accordance with current local
regulations.
General Hygiene Considerations Handle in accordance with good industrial hygiene and safety practice.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
Physical state - Powder
Appearance - Powder
Color - white
Odor Pungent, Slight nitric
pH - <1
Melting point/freezing point - Boiling point / boiling range - Flash point - N/A
Evaporation rate - N/A
Flammability (solid, gas) - Not flammable
Flammability Limit in Air
Upper flammability limit: -
Lower flammability limit: -
Vapor pressure - N/A
Vapor density - N/A
Specific Gravity 1.7
Water solubility Soluble in water
Solubility in other solvents - Partition coefficient - Autoignition temperature - N/A
Decomposition temperature - N/A
Kinematic viscosity - N/A
Dynamic viscosity - N/A
Explosive properties N/A
Oxidizing properties Strong oxidizer and will react vigorously or explosively with many materials including
organic materials, such as wood and paper, and flammable metals.
Other Information
Softening point -
Molecular weight -
VOC Content (%) N/A
Density
Bulk density 48 lb/ft3
SECTION 10. STABILITY AND REACTIVITY
Reactivity
Reacts with water. Strong oxidizer and
will react vigorously or explosively with
many materials including organic
materials, such as wood and paper,
and flammable metals
Chemical stability
Stable under recommended storage conditions.
Possibility of Hazardous Reactions
Reacts with water.
Hazardous polymerization Hazardous polymerization does not occur.
Conditions to avoid
Unintentional contact with water. Heat. Static discharge.
Incompatible materials
Water, alcohols, phenols, and amines. Rubber, coatings, and some plastics. Flammable substances, reducing agents, metal
powders, and organic materials. Reacts with metals to produce heat and corrosive gases.
Hazardous Decomposition Products
Reacts with water to produce nitric acid and heat
SECTION 11. TOXICOLOGICAL INFORMATION
Information on likely routes of exposure
Product Information
Inhalation Product not classified.
Eye contact Causes severe eye damage.
Skin Contact Causes severe skin burns.
Ingestion Harmful if swallowed.
Chemical Name
Hafnium Dinitrate Oxide
CAS 15060-56-7
Oral LD50: > 300 and < 2000 mg/kg bw
Dermal LD50 -
Inhalation LC50 -
Information on toxicological effects
Symptoms May cause skin burns. May cause burning sensation or redness in the eyes. May cause
acute gastrointestinal effects if swallowed.
Delayed and immediate effects as well as chronic effects from short and long-term exposure
Acute toxicity Harmful if swallowed.
Skin corrosion/irritation May cause severe skin burns.
Serious eye damage/eye irritation May cause serious eye damage.
Sensitization Product not classified.
Germ cell mutagenicity Product not classified.
Carcinogenicity Product not classified.
Reproductive toxicity Product not classified.
STOT - single exposure Product not classified.
STOT - repeated exposure Product not classified.
Aspiration hazard Product not classified.
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
This product as shipped is not classified for aquatic toxicity.
Chemical Name Algae/aquatic plants Fish Toxicity to
microorganisms
Crustacea
Hafnium Dinitrate Oxide
15060-56-7
The 72 h EC50 of zirconium
dichloride oxide to
Pseudokirchnerella
subcapitata was 80% v/v
saturated solution.
The 96 h LL50 of zirconium
dinitrate oxide to
Oncorhynchus mykiss was
greater than 100 mg/L.
- The 48 h EC50 of zirconium
dioxide to Daphnia magna
was greater than 100 mg/L.
Persistence and degradability
.Bioaccumulation
Other adverse effects
SECTION 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Disposal of wastes Disposal should be in accordance with applicable regional, national and local laws and
regulations.
Contaminated packaging Disposal should be in accordance with applicable regional, national and local laws and
regulations.
SECTION 14. TRANSPORT INFORMATION
DOT Regulated
UN/ID No. 3085
Proper shipping name Oxidizing Solid, Corrosive, n.o.s. (Hafnium Oxynitrate)
Hazard Class 5.1
Subsidiary class 8
Packing Group II
Special Provisions 62, IB8, IP3, T1, TP33
Emergency Response Guide Number 140
SECTION 15. REGULATORY INFORMATION
International Inventories
TSCA Not Listed
DSL/NDSL Not Listed
EINECS/ELINCS Not Listed
ENCS Not Listed
IECSC Not Listed
KECL Not Listed
PICCS Not Listed
AICS Not Listed
Legend:
TSCA - United States Toxic Substances Control Act Section 8(b) Inventory
DSL/NDSL - Canadian Domestic Substances List/Non-Domestic Substances List
EINECS/ELINCS - European Inventory of Existing Chemical Substances/European List of Notified Chemical Substances
ENCS - Japan Existing and New Chemical Substances
IECSC - China Inventory of Existing Chemical Substances
KECL - Korean Existing and Evaluated Chemical Substances
PICCS - Philippines Inventory of Chemicals and Chemical Substances
AICS - Australian Inventory of Chemical Substances
US Federal Regulations
SARA 313
Section 313 of Title III of the Superfund Amendments and Reauthorization Act of 1986 (SARA). This product does not contain any
chemicals which are subject to the reporting requirements of the Act and Title 40 of the Code of Federal Regulations, Part 372
SARA 311/312 Hazard Categories
Acute health hazard Yes
Chronic Health Hazard No
Fire hazard No
Sudden release of pressure hazard No
Reactive Hazard Yes
CWA (Clean Water Act)
This product does not contain any substances regulated as pollutants pursuant to the Clean Water Act (40 CFR 122.21 and 40
CFR 122.42)
CERCLA
This material, as supplied, does not contain any substances regulated as hazardous substances under the Comprehensive
Environmental Response Compensation and Liability Act (CERCLA) (40 CFR 302) or the Superfund Amendments and
Reauthorization Act (SARA) (40 CFR 355). There may be specific reporting requirements at the local, regional, or state level
pertaining to releases of this material
US State Regulations
California Proposition 65
This product does not contain any Proposition 65 chemicals
U.S. State Right-to-Know Regulations
U.S. EPA Label Information
EPA Pesticide Registration Number Not Applicable
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
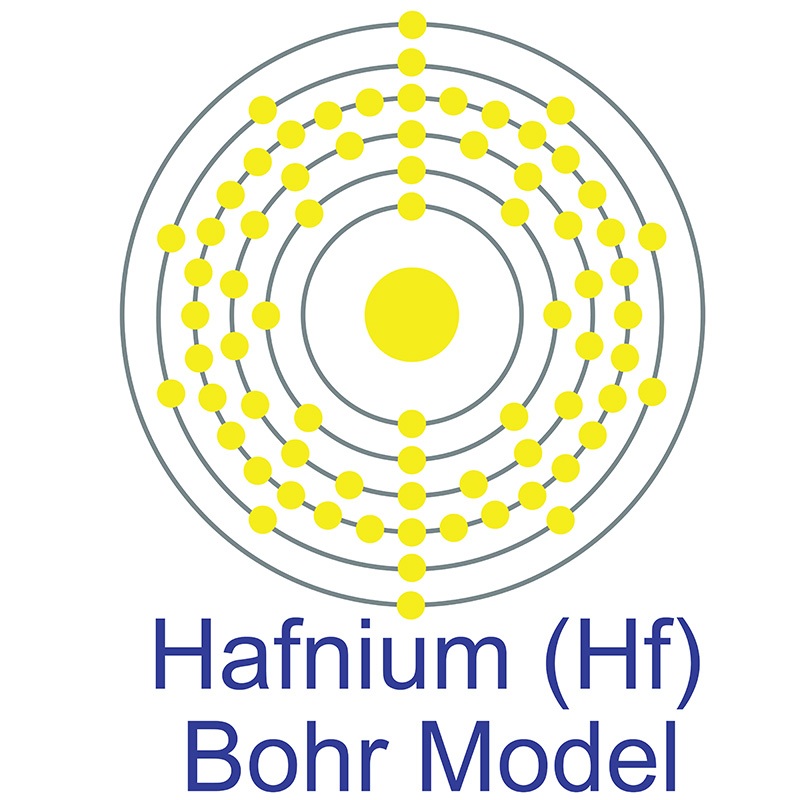 The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electron configuration is [Xe] 4f14 5d2 6s2. The hafnium atom has a radius of 159 pm and a Van der Waals radius of 212 pm. Hafnium was predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy. In its elemental form, hafnium has a lustrous silvery-gray appearance.
The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electron configuration is [Xe] 4f14 5d2 6s2. The hafnium atom has a radius of 159 pm and a Van der Waals radius of 212 pm. Hafnium was predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy. In its elemental form, hafnium has a lustrous silvery-gray appearance.  Hafnium does not exist as a free element in nature. It is found in
Hafnium does not exist as a free element in nature. It is found in