SECTION 1. IDENTIFICATION
Product Name: Potassium Sodium Niobate
Product Number: All applicable American Elements product codes, e.g. NAK-NBO-02
, NAK-NBO-03
, NAK-NBO-04
, NAK-NBO-05
CAS #: 12196-68-8
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Physical hazards Not classified.
Health hazards Skin corrosion/irritation Category 1
Serious eye damage/eye irritation Category 1
Environmental hazards Not classified.
OSHA defined hazards Not classified.


Signal word Danger
Hazard statement Causes severe skin burns and eye damage.
Precautionary statement
Prevention
Do not breathe dust/fume/gas/mist/vapors Wash thoroughly after handling. Wear protective gloves and eye/face protection. Wear protective gloves/protective clothing/eye protection/face protection. In case of inadequate ventilation wear respiratory protection.
Response If swallowed: Rinse mouth. Do NOT induce vomiting. If skin irritation occurs, seek medical advice/attention.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing. If on skin (or hair): Take off immediately all
contaminated clothing. Rinse skin with water/shower. If inhaled: Remove person to fresh air and
keep comfortable for breathing. If in eyes: Rinse cautiously with water for several minutes.
Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a poison
center/doctor. If eye irritation persists: Get medical advice/attention. Take off contaminated
clothing and wash before reuse.
Storage
Store in a well-ventilated place. Keep container tightly closed. Store locked up.
Disposal
Dispose of contents/container in accordance with local/regional/national/international regulations.
Hazard(s) not otherwise classified (HNOC)
None known.
Supplemental information
100% of the mixture consists of component(s) of unknown acute oral toxicity. 100% of the mixture
consists of component(s) of unknown acute dermal toxicity. 100% of the mixture consists of
component(s) of unknown acute hazards to the aquatic environment. 100% of the mixture
consists of component(s) of unknown long-term hazards to the aquatic environment.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Chemical Name / CAS
Potassium Sodium Niobate / 12196-68-8
SECTION 4. FIRST AID MEASURES
Inhalation
Move to fresh air. Call a physician if symptoms develop or persist.
Skin Contact
Take off immediately all contaminated clothing. Rinse skin with water/shower. Call a physician or
poison control center immediately. Chemical burns must be treated by a physician. Wash
contaminated clothing before reuse.
Eye Contact
Immediately flush eyes with plenty of water for at least 15 minutes. Remove contact lenses, if
present and easy to do. Continue rinsing. Call a physician or poison control center immediately.
Ingestion
Call a physician or poison control center immediately. Do not induce vomiting. If vomiting occurs,
keep head low so that stomach content doesn't get into the lungs.
Most important symptoms/effects, acute and delayed
Burning pain and severe corrosive skin damage. Causes serious eye damage. Symptoms may
include stinging, tearing, redness, swelling, and blurred vision. Permanent eye damage including
blindness could result.
Indication of immediate medical attention and special treatment needed
Provide general supportive measures and treat symptomatically. Chemical burns: Flush with water
immediately. While flushing, remove clothes which do not adhere to affected area. Call an
ambulance. Continue flushing during transport to hospital. Keep victim under observation.
Symptoms may be delayed
General Information
Ensure that medical personnel are aware of the material(s) involved, and take precautions to
protect themselves.
SECTION 5. FIREFIGHTING MEASURES
Suitable extinguishing media Powder. Dry sand.
Unsuitable extinguishing media Water.
Specific hazards arising from the chemical During fire, gases hazardous to health may be formed.
Special protective equipment and precautions for firefighters Self-contained breathing apparatus and full protective clothing must be worn in case of fire.
Fire fighting equipment/instructions Move containers from fire area if you can do so without risk.
Specific methods Use standard firefighting procedures and consider the hazards of other involved materials.
General fire hazards No unusual fire or explosion hazards noted.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Keep unnecessary personnel away. Keep people away from and upwind of spill/leak. Wear
appropriate protective equipment and clothing during clean-up. Do not touch damaged containers
or spilled material unless wearing appropriate protective clothing. Ensure adequate ventilation.
Local authorities should be advised if significant spillages cannot be contained.
Methods and materials for containment and cleaning up
Large Spills: Stop the flow of material, if this is without risk. Dike the spilled material, where this is
possible. Absorb in vermiculite, dry sand or earth and place into containers. Following product
recovery, flush area with water.
Small Spills: Wipe up with absorbent material (e.g. cloth, fleece). Clean surface thoroughly to
remove residual contamination.
Never return spills to original containers for re-use.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling
Do not get in eyes, on skin, or on clothing. Provide adequate ventilation. Wear appropriate
personal protective equipment.
Conditions for safe storage, including any incompatibilities
Store in original tightly closed container.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Occupational exposure limits This mixture has no ingredients that have PEL, TLV, or other recommended exposure limit.
Biological limit values No biological exposure limits noted for the ingredient(s).
Appropriate engineering controls Eye wash facilities and emergency shower must be available when handling this product.
Individual protection measures, such as personal protective equipment
Eye/face protection Wear safety glasses with side shields (or goggles) and a face shield.
Skin protection
Hand protection Wear appropriate chemical resistant gloves.
Other Wear appropriate chemical resistant clothing.
Respiratory protection In case of insufficient ventilation, wear suitable respiratory equipment.
Thermal hazards Wear appropriate thermal protective clothing, when necessary.
General hygiene considerations
Always observe good personal hygiene measures, such as washing after handling the material
and before eating, drinking, and/or smoking.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance
Physical state Solid.
Form Solid.
Color Not available.
Odor Not available.
Odor threshold Not available.
pH Not available.
Melting point/freezing point 2012 °F (1100 °C) / 2012 °F (1100 °C) estimated
Initial boiling point and boiling
range
Not available.
Flash point Not available.
Evaporation rate Not available.
Flammability (solid, gas) Not available.
Upper/lower flammability or explosive limits
Flammability limit - lower (%) Not available.
Flammability limit - upper (%) Not available.
Explosive limit - lower (%) Not available.
Explosive limit - upper (%) Not available.
Vapor pressure Not available.
Vapor density Not available.
Relative density Not available.
Solubility(ies)
Solubility (water) Not available.
Partition coefficient (n-octanol/water) Not available.
Auto-ignition temperature Not available.
Decomposition temperature Not available.
Viscosity Not available.
Other information
Explosive properties Not explosive.
Oxidizing properties Not oxidizing.
Specific gravity 4.51 g/cc
SECTION 10. STABILITY AND REACTIVITY
Reactivity The product is stable and non-reactive under normal conditions of use, storage and transport.
Chemical stability Material is stable under normal conditions.
Possibility of hazardous reactions Hazardous polymerization does not occur.
Conditions to avoid Contact with incompatible materials.
Incompatible materials Strong oxidizing agents.
Hazardous decomposition products No hazardous decomposition products are known.
SECTION 11. TOXICOLOGICAL INFORMATION
Information on likely routes of exposure
Inhalation May cause irritation to the respiratory system.
Skin contact Causes severe skin burns.
Eye contact Causes serious eye damage.
Ingestion Causes digestive tract burns.
Symptoms related to the physical, chemical and toxicological characteristics
Burning pain and severe corrosive skin damage. Causes serious eye damage. Symptoms may
include stinging, tearing, redness, swelling, and blurred vision. Permanent eye damage including
blindness could result.
Information on toxicological effects
Acute toxicity Not known.
Skin corrosion/irritation Causes severe skin burns and eye damage.
Serious eye damage/eye irritation Causes serious eye damage
Respiratory or skin sensitization
Respiratory sensitization Not a respiratory sensitizer.
Skin sensitization This product is not expected to cause skin sensitization.
Germ cell mutagenicity
No data available to indicate product or any components present at greater than 0.1% are
mutagenic or genotoxic.
Carcinogenicity Not classifiable as to carcinogenicity to humans.
IARC Monographs. Overall Evaluation of Carcinogenicity
Not listed.
OSHA Specifically Regulated Substances (29 CFR 1910.1001-1052)
Not regulated.
US. National Toxicology Program (NTP) Report on Carcinogens
Not listed.
Reproductive toxicity This product is not expected to cause reproductive or developmental effects.
Specific target organ toxicity -
single exposure
Not classified.
Specific target organ toxicity -
repeated exposure
Not classified.
Aspiration hazard Not an aspiration hazard.
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
The product is not classified as environmentally hazardous. However, this does not exclude the
possibility that large or frequent spills can have a harmful or damaging effect on the environment.
Persistence and degradability
Bioaccumulative potential No data available.
Mobility in soil No data available.
Other adverse effects No other adverse environmental effects (e.g. ozone depletion, photochemical ozone creation
potential, endocrine disruption, global warming potential) are expected from this component.
SECTION 13. DISPOSAL CONSIDERATIONS
Disposal Instructions
Collect and reclaim or dispose in sealed containers at licensed waste disposal site. Dispose of
contents/container in accordance with local/regional/national/international regulations.
Local disposal regulations Dispose in accordance with all applicable regulations.
Hazardous waste code
The waste code should be assigned in discussion between the user, the producer and the waste
disposal company
Waste from residues / unused products
Dispose of in accordance with local regulations. Empty containers or liners may retain some
product residues. This material and its container must be disposed of in a safe manner (see:
Disposal instructions).
Contaminated Packaging
Since emptied containers may retain product residue, follow label warnings even after container is
emptied. Empty containers should be taken to an approved waste handling site for recycling or
disposal.
SECTION 14. TRANSPORT INFORMATION
DOT
Not regulated as dangerous goods.
IATA
Not regulated as dangerous goods.
IMDG
Not regulated as dangerous goods.
SECTION 15. REGULATORY INFORMATION
US federal regulations
Toxic Substances Control Act (TSCA)
TSCA Section 12(b) Export Notification (40 CFR 707, Subpt. D)
Not regulated.
CERCLA Hazardous Substance List (40 CFR 302.4)
Sodium (CAS 7440-23-5) Listed.
SARA 304 Emergency release notification
Not regulated.
OSHA Specifically Regulated Substances (29 CFR 1910.1001-1052)
Not regulated.
SARA 302 Extremely hazardous substance
Superfund Amendments and Reauthorization Act of 1986 (SARA)
Not listed.
SARA 311/312 Hazardous Yes
chemical
Skin corrosion or irritation
Serious eye damage or eye irritation
Classified hazard
categories
SARA 313 (TRI reporting)
Not regulated.
Other federal regulations
Clean Air Act (CAA) Section 112 Hazardous Air Pollutants (HAPs) List
Not regulated.
Clean Air Act (CAA) Section 112(r) Accidental Release Prevention (40 CFR 68.130)
Not regulated.
Safe Drinking Water Act Not regulated.
(SDWA)
California Safe Drinking Water and Toxic Enforcement Act of 1986 (Proposition 65): This material
is not known to contain any chemicals currently listed as carcinogens or reproductive toxins.
US state regulations
California Proposition 65
US. California. Candidate Chemicals List. Safer Consumer Products Regulations (Cal. Code Regs, tit. 22, 69502.3, subd. (a))
Sodium (CAS 7440-23-5)
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
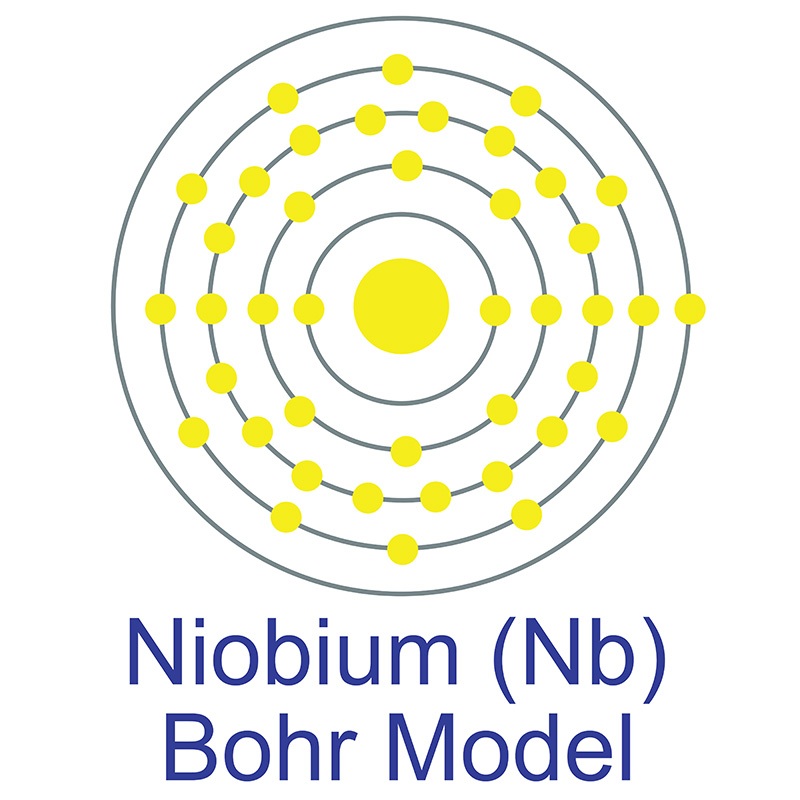 The number of electrons in each of niobium's shells is 2, 8, 18, 12, 1 and its electron configuration is [Kr] 4d4 5s1. The niobium atom has a radius of 146 pm and a Van der Waals radius of 207 pm. Niobium was discovered by Charles Hatchett in 1801 and first isolated by Christian Wilhelm Blomstrand in 1864. In its elemental form, niobium has a gray metallic appearance. Niobium has the largest magnetic penetration depth of any element and is one of three elemental type-II superconductors (
The number of electrons in each of niobium's shells is 2, 8, 18, 12, 1 and its electron configuration is [Kr] 4d4 5s1. The niobium atom has a radius of 146 pm and a Van der Waals radius of 207 pm. Niobium was discovered by Charles Hatchett in 1801 and first isolated by Christian Wilhelm Blomstrand in 1864. In its elemental form, niobium has a gray metallic appearance. Niobium has the largest magnetic penetration depth of any element and is one of three elemental type-II superconductors ( along with
along with  See more Potassium products.
See more Potassium products.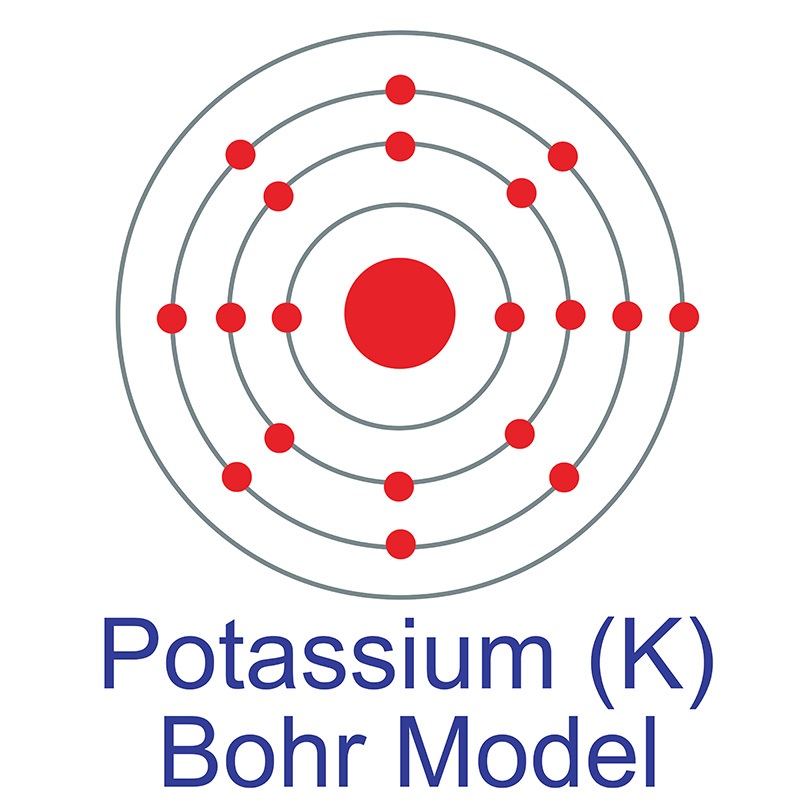 In its elemental form, potassium has a silvery gray metallic appearance, but its
In its elemental form, potassium has a silvery gray metallic appearance, but its 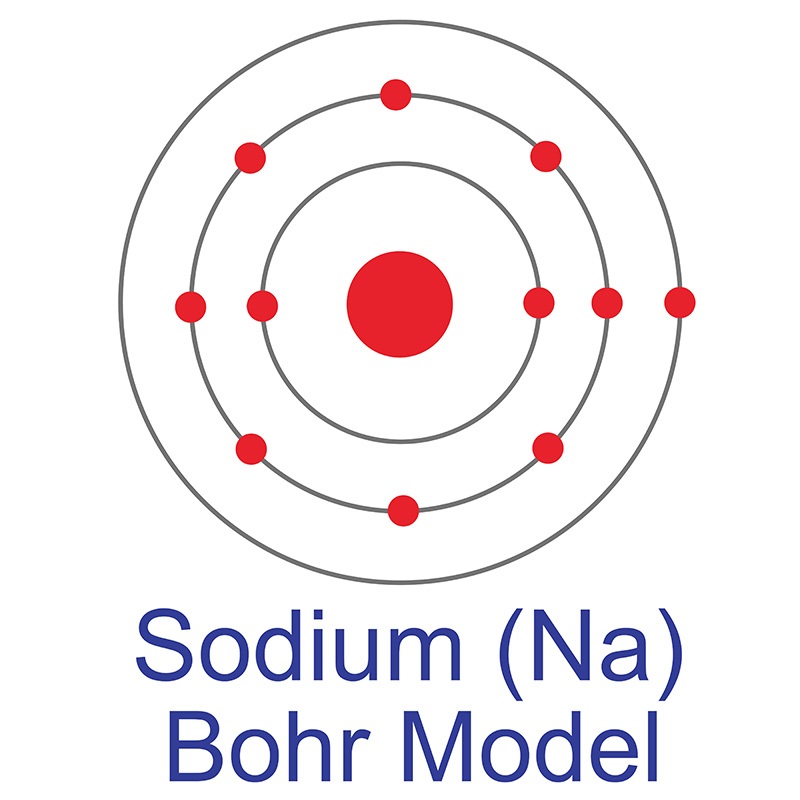 See more Sodium products.
See more Sodium products.