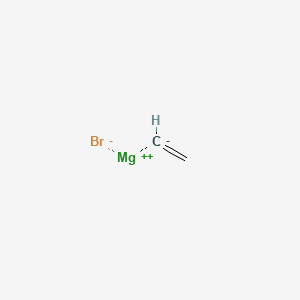SECTION 1. IDENTIFICATION
Product Name: Vinylmagnesium Bromide Solution
Product Number: All applicable American Elements product codes, e.g. MG-OMX-01-SOL.1MTHF
CAS #: 1826-67-1
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification
Classification under 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200)
Flammable liquids Category 1
Substances/mixtures which, in contact with water, emit
flammable gases
Category 1
Skin Corrosion/irritation Category 1 B
Serious Eye Damage/Eye Irritation Category 1
Specific target organ toxicity (single exposure) Category 3
Target Organs - Respiratory system.
Label Elements
Signal Word
Danger
Hazard Statements
Extremely flammable liquid and vapor
In contact with water releases flammable gases which may ignite spontaneously
Causes severe skin burns and eye damage
May cause respiratory irritation
Precautionary Statements
Prevention
Do not breathe dust/fume/gas/mist/vapors/spray
Wash face, hands and any exposed skin thoroughly after handling
Wear protective gloves/protective clothing/eye protection/face protection
Use only outdoors or in a well-ventilated area
Keep away from heat/sparks/open flames/hot surfaces. - No smoking
Keep container tightly closed
Ground/bond container and receiving equipment
Use explosion-proof electrical/ventilating/lighting/equipment
Use only non-sparking tools
Take precautionary measures against static discharge
Keep away from any possible contact with water, because of violent reaction and possible flash fire
Handle under inert gas. Protect from moisture
Keep cool
Response
Immediately call a POISON CENTER or doctor/physician
Inhalation
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
Skin
IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower
Wash contaminated clothing before reuse
Brush off loose particles from skin. Immerse in cool water/wrap with wet bandages
Eyes
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
Ingestion
IF SWALLOWED: Rinse mouth. DO NOT induce vomiting
Fire
In case of fire: Use CO2, dry chemical, or foam for extinction
Storage
Store locked up
Store in a well-ventilated place. Keep container tightly closed
Store in a dry place. Store in a closed container
Disposal
Dispose of contents/container to an approved waste disposal plant
Hazards not otherwise classified (HNOC)
Reacts violently with water
May form explosive peroxides
Unknown Acute Toxicity
10 % of the mixture consists of ingredients of unknown toxicity.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component CAS-No Weight %
Tetrahydrofuran 109-99-9 90
Magnesium, bromoethenyl- 1826-67-1 10
SECTION 4. FIRST AID MEASURES
Eye Contact Immediate medical attention is required. Rinse immediately with plenty of water, also under
the eyelids, for at least 15 minutes.
Skin Contact Wash off immediately with plenty of water for at least 15 minutes. Immediate medical
attention is required.
Inhalation Move to fresh air. If breathing is difficult, give oxygen. Get medical attention immediately if
symptoms occur.
Ingestion Do not induce vomiting. Obtain medical attention.
Most important symptoms/effects Breathing difficulties. Causes burns by all exposure routes. Inhalation of high vapor
concentrations may cause symptoms like headache, dizziness, tiredness, nausea and
vomiting: Product is a corrosive material. Use of gastric lavage or emesis is
contraindicated. Possible perforation of stomach or esophagus should be investigated:
Ingestion causes severe swelling, severe damage to the delicate tissue and danger of
perforation
Notes to Physician Treat symptomatically
SECTION 5. FIREFIGHTING MEASURES
Suitable Extinguishing Media Dry chemical. approved class D extinguishers. clay. sodium carbonate.
Unsuitable Extinguishing Media No data available
Flash Point -17 °C / 1.4 °F
Method - No data available
Autoignition Temperature No data available
Explosion Limits
Upper No data available
Lower No data available
Sensitivity to Mechanical Impact No data available
Sensitivity to Static Discharge No data available
Specific Hazards Arising from the Chemical
Flammable. Contact with water liberates toxic gas. Water reactive. Produce flammable gases on contact with water.
Hazardous Combustion Products
Carbon monoxide (CO) Carbon dioxide (CO2) Fumes Magnesium oxides
Protective Equipment and Precautions for Firefighters
As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full
protective gear.
NFPA
Health 3
Flammability 4
Instability 0
Physical hazard W
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal Precautions Ensure adequate ventilation. Use personal protective equipment.
Environmental Precautions See Section 12 for additional ecological information.
Methods for Containment and Clean Up
Soak up with inert absorbent material (e.g. sand, silica gel, acid binder, universal binder,
sawdust). Keep in suitable, closed containers for disposal. Remove all sources of ignition.
Use spark-proof tools and explosion-proof equipment. Do not expose spill to water.
SECTION 7. HANDLING AND STORAGE
Handling Do not breathe vapors or spray mist. Do not get in eyes, on skin, or on clothing. Use only in
area provided with appropriate exhaust ventilation. Use explosion-proof equipment. Use
only non-sparking tools. Do not allow contact with water because of violent reaction. Keep
away from open flames, hot surfaces and sources of ignition. To avoid ignition of vapors by
static electricity discharge, all metal parts of the equipment must be grounded. Take
precautionary measures against static discharges. If peroxide formation is suspected, do
not open or move container.
Storage Keep in a dry place. Keep container tightly closed. Keep away from heat and sources of
ignition. Keep away from direct sunlight. Store at room temperature. Never allow product to
get in contact with water during storage. Corrosives area. Flammables area. Keep under
nitrogen. Keep container tightly closed in a dry and well-ventilated place. Containers should
be dated when opened and tested periodically for the presence of peroxides. Should
crystals form in a peroxidizable liquid, peroxidation may have occurred and the product
should be considered extremely dangerous. In this instance, the container should only be
opened remotely by professionals.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Exposure Guidelines
Component ACGIH TLV OSHA PEL NIOSH IDLH
Tetrahydrofuran TWA: 50 ppm
STEL: 100 ppm
Skin
(Vacated) TWA: 200 ppm
(Vacated) TWA: 590 mg/m3
(Vacated) STEL: 250 ppm
(Vacated) STEL: 735 mg/m3
TWA: 200 ppm
TWA: 590 mg/m3
IDLH: 2000 ppm
TWA: 200 ppm
TWA: 590 mg/m3
STEL: 250 ppm
STEL: 735 mg/m3
Component Quebec Mexico OEL (TWA) Ontario TWAEV
Tetrahydrofuran TWA: 100 ppm
TWA: 300 mg/m3
TWA: 200 ppm
TWA: 590 mg/m3
STEL: 250 ppm
STEL: 735 mg/m3
TWA: 50 ppm
STEL: 100 ppm
Skin
Legend
ACGIH - American Conference of Governmental Industrial Hygienists
OSHA - Occupational Safety and Health Administration
NIOSH IDLH: The National Institute for Occupational Safety and Health Immediately Dangerous to Life or Health
Engineering Measures Use explosion-proof electrical/ventilating/lighting/equipment. Ensure that eyewash stations
and safety showers are close to the workstation location.
Personal Protective Equipment
Eye/face Protection Wear appropriate protective eyeglasses or chemical safety goggles as described by
OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard
EN166.
______________________________________________________________________________________________
Skin and body protection Wear appropriate protective gloves and clothing to prevent skin exposure.
Respiratory Protection
Page 4 / 9
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard
EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if
exposure limits are exceeded or if irritation or other symptoms are experienced.
Hygiene Measures Handle in accordance with good industrial hygiene and safety practice.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State Liquid
Appearance Amber
Odor pungent
Odor Threshold No data available
pH No data available
Melting Point/Range No data available
Boiling Point/Range No data available
Flash Point -17 °C / 1.4 °F
Evaporation Rate No data available
Flammability (solid,gas) No data available
Flammability or explosive limits
Upper No data available
Lower No data available
Vapor Pressure No data available
Vapor Density No data available
Relative Density 0.980
Solubility No data available
Partition coefficient; n-octanol/water No data available
Autoignition Temperature No data available
Decomposition Temperature No data available
Viscosity No data available
Molecular Formula C2 H3 Br Mg
Molecular Weight 131.25
SECTION 10. STABILITY AND REACTIVITY
Reactive Hazard Yes
Stability May form explosive peroxides. Moisture sensitive. Air sensitive. Light sensitive. Reacts
violently with water, liberating highly flammable gases.
Conditions to Avoid Excess heat. Exposure to air. Exposure to light. Incompatible products. Exposure to moist
air or water.
Incompatible Materials Acids, Bases, Water, Alcohols
Hazardous Decomposition Products Carbon monoxide (CO), Carbon dioxide (CO2), Fumes, Magnesium oxides
Hazardous Polymerization Hazardous polymerization does not occur.
Hazardous Reactions Reacts violently with water, liberating highly flammable gases.
SECTION 11. TOXICOLOGICAL INFORMATION
Acute Toxicity
Product Information No acute toxicity information is available for this product
Oral LD50 Based on ATE data, the classification criteria are not met. ATE > 2000 mg/kg.
Dermal LD50 Based on ATE data, the classification criteria are not met. ATE > 2000 mg/kg.
Vapor LC50 Based on ATE data, the classification criteria are not met. ATE > 20 mg/l.
Component Information
Component LD50 Oral LD50 Dermal LC50 Inhalation
Tetrahydrofuran 1650 mg/kg ( Rat ) > 2000 mg/kg (Rabbit) 180 mg/L ( Rat ) 1 h
53.9 mg/L ( Rat ) 4 h
Toxicologically Synergistic
Products
No data available
Delayed and immediate effects as well as chronic effects from short and long-term exposure
Irritation Causes severe irritation and or burns
Sensitization No data available
Carcinogenicity Possible cancer hazard. May cause cancer based on animal data.
Component CAS-No IARC NTP ACGIH OSHA Mexico
Tetrahydrofuran 109-99-9 Not listed Not listed A3 Not listed Not listed
Magnesium,
bromoethenyl-
1826-67-1 Not listed Not listed Not listed Not listed Page 5 / 9
Mutagenic Effects No data available
Reproductive Effects No data available.
Developmental Effects No data available.
Teratogenicity No data available.
STOT - single exposure Respiratory system
STOT - repeated exposure None known
Aspiration hazard No data available
Symptoms / effects,both acute and
delayed
Inhalation of high vapor concentrations may cause symptoms like headache, dizziness,
tiredness, nausea and vomiting: Product is a corrosive material. Use of gastric lavage or
emesis is contraindicated. Possible perforation of stomach or esophagus should be
investigated: Ingestion causes severe swelling, severe damage to the delicate tissue and
danger of perforation
Endocrine Disruptor Information
Component EU - Endocrine Disrupters
Candidate List
EU - Endocrine Disruptors -
Evaluated Substances
Japan - Endocrine Disruptor
Information
Tetrahydrofuran Group III Chemical N/A N/A
Other Adverse Effects The toxicological properties have not been fully investigated. See actual entry in RTECS for
complete information
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
.
Component Freshwater Algae Freshwater Fish Microtox Water Flea
Tetrahydrofuran Not listed 2160 mg/l LC50 = 96 h
Pimephales promelas
Leuciscus idus: LC50: 2820
mg/L/48h
Not listed EC50 48 h 3485 mg/l
EC50: >10000 mg/L/24h
Persistence and Degradability No data available
Bioaccumulation/ Accumulation No data available.
Mobility .
Component log Pow
Tetrahydrofuran 0.45
SECTION 13. DISPOSAL CONSIDERATIONS
Waste Disposal Methods Chemical waste generators must determine whether a discarded chemical is classified as a
hazardous waste. Chemical waste generators must also consult local, regional, and
national hazardous waste regulations to ensure complete and accurate classification.
Component RCRA - U Series Wastes RCRA - P Series Wastes
Tetrahydrofuran - 109-99-9 U213 -
SECTION 14. TRANSPORT INFORMATION
DOT
UN-No UN3399
Proper Shipping Name ORGANOMETALLIC SUBSTANCE, LIQUID, WATER-REACTIVE, FLAMMABLE
Hazard Class 4.3
Subsidiary Hazard Class 3, 8
Packing Group I
TDG
UN-No UN3399
Proper Shipping Name ORGANOMETALLIC SUBSTANCE, LIQUID, WATER-REACTIVE, FLAMMABLE
Hazard Class 4.3
Subsidiary Hazard Class 3, 8
Packing Group I
IATA
UN-No UN3399
Proper Shipping Name ORGANOMETALLIC SUBSTANCE, LIQUID, WATER-REACTIVE, FLAMMABLE
Hazard Class 4.3
Subsidiary Hazard Class 3
Packing Group I
IMDG/IMO
UN-No UN3399
Proper Shipping Name ORGANOMETALLIC SUBSTANCE, LIQUID, WATER-REACTIVE, FLAMMABLE
Hazard Class 4.3
Subsidiary Hazard Class 3
Packing Group
SECTION 15. REGULATORY INFORMATION
International Inventories
Component TSCA DSL NDSL EINECS ELINCS NLP PICCS ENCS AICS IECSC KECL
Tetrahydrofuran X X - 203-726-8 - X X X X X
Magnesium, bromoethenyl- X - X 217-375-3 - - X - - X
Legend:
X - Listed
E - Indicates a substance that is the subject of a Section 5(e) Consent order under TSCA.
F - Indicates a substance that is the subject of a Section 5(f) Rule under TSCA.
N - Indicates a polymeric substance containing no free-radical initiator in its inventory name but is considered to cover the designated
polymer made with any free-radical initiator regardless of the amount used.
P - Indicates a commenced PMN substance
R - Indicates a substance that is the subject of a Section 6 risk management rule under TSCA.
S - Indicates a substance that is identified in a proposed or final Significant New Use Rule
T - Indicates a substance that is the subject of a Section 4 test rule under TSCA.
XU - Indicates a substance exempt from reporting under the Inventory Update Rule, i.e. Partial Updating of the TSCA Inventory Data Base
Production and Site Reports (40 CFR 710(B).
Y1 - Indicates an exempt polymer that has a number-average molecular weight of 1,000 or greater.
Y2 - Indicates an exempt polymer that is a polyester and is made only from reactants included in a specified list of low concern reactants
that comprises one of the eligibility criteria for the exemption rule.
U.S. Federal Regulations
TSCA 12(b)
Component TSCA 12(b)
Tetrahydrofuran Section 4, 1 % de minimus concentration
SARA 313 N/A
SARA 311/312 Hazardous Categorization
Acute Health Hazard Yes
Chronic Health Hazard No
Fire Hazard Yes
Sudden Release of Pressure Hazard No
Reactive Hazard Yes
Clean Water Act
N/A
Clean Air Act
N/A
OSHA Occupational Safety and Health Administration
N/A
CERCLA
This material, as supplied, contains one or more substances regulated as a hazardous substance under the Comprehensive
Environmental Response Compensation and Liability Act (CERCLA) (40 CFR 302)
Component Hazardous Substances RQs CERCLA EHS RQs
Tetrahydrofuran 1000 lb -
California Proposition 65 This product does not contain any Proposition 65 chemicals
State Right-to-Know
Component Massachusetts New Jersey Pennsylvania Illinois Rhode Island
Tetrahydrofuran X X X - X
U.S. Department of Transportation
Reportable Quantity (RQ): N
DOT Marine Pollutant N
DOT Severe Marine Pollutant N
U.S. Department of Homeland Security
This product does not contain any DHS chemicals.
Other International Regulations
Mexico - Grade No data available
Canada
This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations (CPR) and
the MSDS contains all the information required by the CPR
WHMIS Hazard Class B2 Flammable liquid
B6 Reactive flammable material
E Corrosive material
F Dangerously reactive material
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 See more Magnesium products.
See more Magnesium products. In its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. Commercially, magnesium is primarily used in the creation of strong and lightweight
In its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. Commercially, magnesium is primarily used in the creation of strong and lightweight