SECTION 1. IDENTIFICATION
Product Name: Zinc Benzenesulfinate Dihydrate
Product Number: All applicable American Elements product codes, e.g. ZN-BZSFNAT-02-C.2HYD
, ZN-BZSFNAT-03-C.2HYD
, ZN-BZSFNAT-04-C.2HYD
, ZN-BZSFNAT-05-C.2HYD
CAS #: 24308-84-7
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
GHS Classification
No data available.
Pictogram
No data available.
Signal word
No data available.
Hazard statement(s)
No data available.
Precautionary statement(s)
No data available.
Hazards not otherwise classified (HNOC) or not covered by GHS
No data available.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Synonyms:
No data available.
CAS#:
[24308-84-7]
Purity:
98%
EC#:
246-148-1
SECTION 4. FIRST AID MEASURES
General information: Immediately remove any clothing contaminated by the product. Move out of dangerous area. Consult a physician and show this safety data sheet.
Inhalation: Move person to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Obtain medical aid.
Skin contact: Immediately flush skin with running water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Obtain medical aid immediately.
Eye contact: Immediately flush open eyes with running water for at least 15 minutes. Obtain medical aid immediately.
Ingestion: Do NOT induce vomiting without medical advice. Rinse mouth with water. Never administer anything by mouth to an unconscious person. Obtain medical aid immediately.
Most important symptoms and effects, both acute and delayed: No further information available. Please see headings 2 and 11.
Indication of any immediate medical attention and special treatment needed: No further information available.
SECTION 5. FIREFIGHTING MEASURES
Suitable extinguishing media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Specific hazards arising from the chemical: Sulfur oxides, Carbon oxides, Zinc oxides
Advice for firefighters: As in any fire, wear a MSHA/NIOSH-approved or equivalent, pressure-demand, self-contained breathing apparatus and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures: Use personal protective equipment.and keep unprotected personnel away. Ensure adequate ventilation. Remove all sources of ignition. Prevent further leak or spill if safe to do so. For personal protective equipment, please refer to heading 8.
Environmental precautions: Do not let product enter drains, other waterways, or soil.
Methods and materials for containment and cleaning up: Prevent further leak or spill if safe to do so. Vacuum, sweep up, or absorb with inert material and place into a suitable disposal container. Consult local regulations for disposal. Also, see heading 13.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling: Avoid contact with skin, eyes, and personal clothing. Wash hands thoroughly after handling. Avoid breathing fumes. Use only with adequate ventilation. Wear suitable protective clothing, gloves, and eye/face protection. Keep away from sources of ignition. Minimize dust generation and accumulation. Keep container tightly closed. Open and handle container with care. Do not eat, drink, or smoke while handling.
Conditions for safe storage, including any incompatibilities: Store in a tightly-closed container when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep away from sources of ignition.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Exposure limits
OSHA PEL:
No data available.
NIOSH REL:
No data available.
ACGIH TLV:
No data available.
Appropriate engineering controls: Avoid contact with skin, eyes, and clothing. Wash hands before breaks and immediately after handling the product. Facilities storing or utilizing this material should be equipped with an eyewash fountain. Use adequate ventilation to keep airborne concentrations low.
Personal protection
Eyes: Wear chemical splash goggles.
Hand: Wear protective gloves.
Skin and body: Wear protective lab coat and boots.
Respiratory: Use NIOSH/MSHA or CEN approved respirator.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State:
No data available.
Molecular Formula:
Zn(C6H5O2S)2.2(H2O)
Molecular Weight:
383.756
Odor:
No data available.
pH:
No data available.
Boiling Point Range:
No data available.
Freezing/Melting Point:
217-221°C
Flash Point:
No data available.
Evaporation Rate:
No data available.
Flammability (solid, gas):
Please see section 2.
Explosive limits:
No data available.
Vapor Pressure:
No data available.
Vapor Density:
No data available.
Solubility:
No data available.
Relative Density:
No data available.
Refractive Index:
No data available.
Volatility:
No data available.
Auto-ignition temperature:
No data available.
Decomposition Temperature:
No data available.
SECTION 10. STABILITY AND REACTIVITY
Reactivity
No data available.
Chemical stability
Stable under recommended temperatures and pressures.
Possibility of hazardous reactions
No data available.
Conditions to avoid
Dust generation.
Incompatible materials
Strong oxidizing agents.
Hazardous decomposition products
Sulfur oxides, Carbon oxides, Zinc oxides
SECTION 11. TOXICOLOGICAL INFORMATION
RTECS#
No data available.
Acute toxicity
No data available.
Routes of exposure
Inhalation, eye contact, skin contact, ingestion.
Symptoms related to the physical, chemical and toxicological characteristics
Skin contact may result in inflammation characterized by itching, scaling, reddening, blistering, pain or dryness. Eye contact may result in redness, pain or severe eye damage. Inhalation may cause irritation of the lungs and respiratory system. Overexposure may result in serious illness or death.
Carcinogenicity
IARC
Not classified.
NTP
Not listed.
OSHA
Not listed.
Acute toxic effects
Inflammation of the eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening, or, occasionally, blistering.
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
No data available.
Persistence and degradability
No data available.
Bioaccumulative potential
No data available.
Mobility in soil
No data available.
Other adverse effects
No data available.
SECTION 13. DISPOSAL CONSIDERATIONS
Disposal of waste: Chemical waste generators must determine whether a discarded chemical is classified as hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR 261.3. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate classification. Observe all federal, state and local regulations when disposing of the substance.
Disposal of packaging: Do not reuse containers. Dispose of as unused product.
SECTION 14. TRANSPORT INFORMATION
DOT (U.S.)
UN number
N/A
UN proper shipping name
N/A
Transport hazard class(es)
N/A
Packing group
N/A
SECTION 15. REGULATORY INFORMATION
TSCA Chemical Inventory:
This product is on the EPA Toxic Substance Control Act (TSCA) inventory. The product is supplied solely for use in research and development by or under the supervision of a technically qualified individual as defined in 40 CFR 720 et seq. The health risks have not been fully determined. Any information that is or becomes available will be supplied on an SDS sheet.
California Proposition 65:
Not listed.
EC#:
246-148-1
NFPA rating:
Health:
Flammability:
Instability:
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
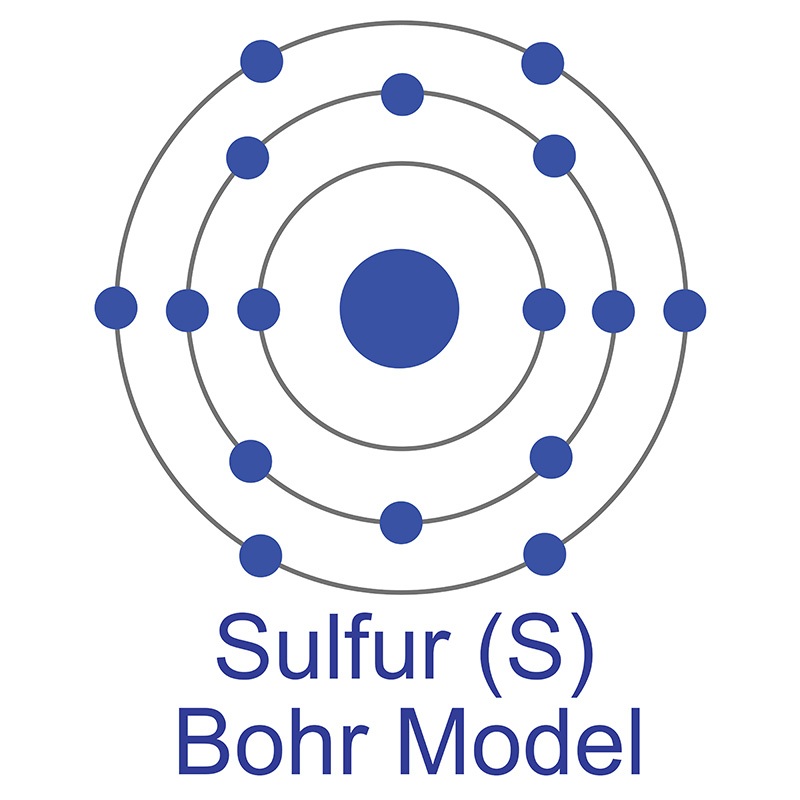 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.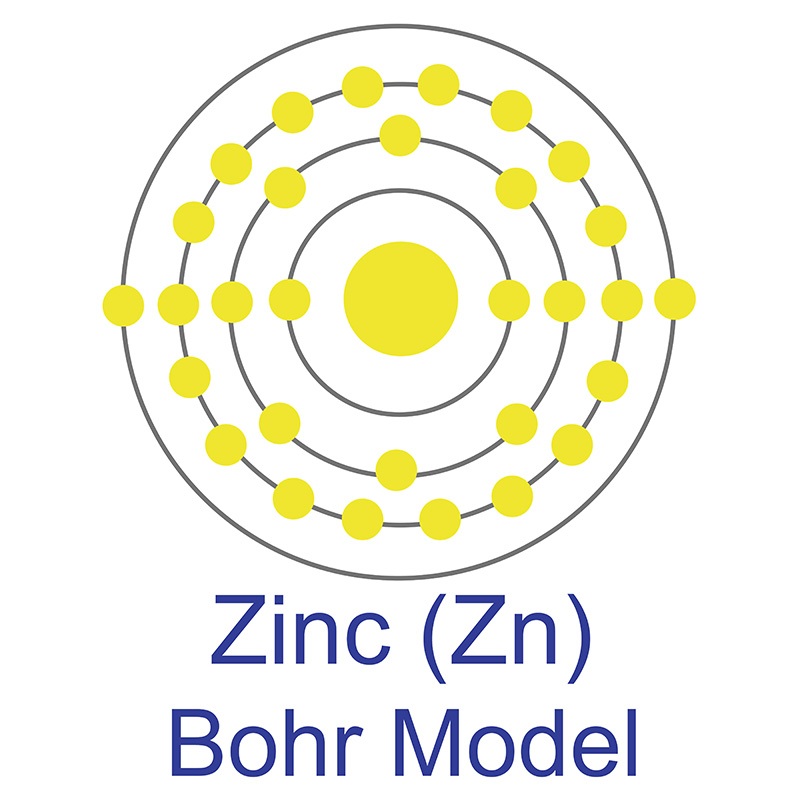 The zinc atom has a radius of 134 pm and a Van der Waals radius of 210 pm. Zinc was discovered by Indian
The zinc atom has a radius of 134 pm and a Van der Waals radius of 210 pm. Zinc was discovered by Indian  It is a fair conductor of electricity, and burns in air at high red producing white clouds of the
It is a fair conductor of electricity, and burns in air at high red producing white clouds of the