About Roentgenium
Roentgenium, a synthetic transactinide (or super heavy element), was first synthesized in 1994 at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung (GSI)) in Darmstadt, Germany. In 2004, IUPAC accepted the GSI’s suggestion of the name roentgenium in honor of Wilhelm Conrad Röntgen. In 1895, Röntgen recognized x-rays as a unique type of rays with special properties that made them uniquely useful in a variety of applications. Roentgenium itself, however, has no commercial applications and much remains unknown about its properties due to its extremely short half-life.
Roentgenium Properties
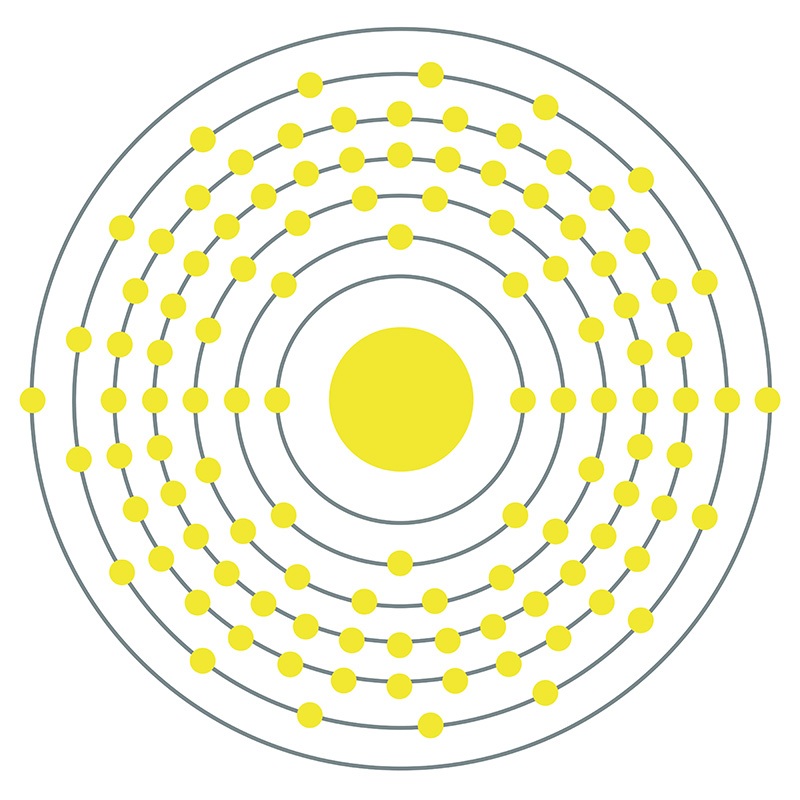
 Roentgenium is a D-Block, Group 11, Period 7 element. The number of electrons in each of Roentgenium's shells is 2, 8, 18, 32, 32, 18, 2 and its electron configuration is [Rn] 5f14 6d10 7s1. In its elemental form, Roentgenium's CAS number is 54386-24-2. Roentgenium was discovered in 1994 at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung (GSI)) in Darmstadt, Germany by Sigurd Hofmann, Peter Armbruster and Gottfried Münzenberg. Roentgenium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses. Roentgenium is named after Wilhelm Röntgen, the physicist who discovered X-rays.
Roentgenium is a D-Block, Group 11, Period 7 element. The number of electrons in each of Roentgenium's shells is 2, 8, 18, 32, 32, 18, 2 and its electron configuration is [Rn] 5f14 6d10 7s1. In its elemental form, Roentgenium's CAS number is 54386-24-2. Roentgenium was discovered in 1994 at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung (GSI)) in Darmstadt, Germany by Sigurd Hofmann, Peter Armbruster and Gottfried Münzenberg. Roentgenium is a synthetic element that is not present in the environment. Little is known about the element, its appearance is unknown, and it has no known uses. Roentgenium is named after Wilhelm Röntgen, the physicist who discovered X-rays.
Roentgenium information, including technical data, properties, and other useful facts are specified below. Scientific facts such as the atomic structure, ionization energy, abundance on Earth, conductivity, and thermal properties are included.
Roentgenium Isotopes
Roentgenium (Rg) is an artificial element. Like all artificial elements, it has no stable isotopes.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 272Rg | 272.15362(36)# | 2.0(8) ms [3.8(+14-8) ms] | a to 268Mt | 5+#,6+# | N/A | 1933.11 | - |
| 273Rg | 273.15368(65)# | 5# ms | Unknown | N/A | N/A | 1941.19 | - |
| 274Rg | 274.15571(66)# | 6.4(+307-29) ms | a to 270Mt | N/A | N/A | 1949.27 | - |
| 275Rg | 275.15614(74)# | 10# ms | Unknown | N/A | N/A | 1957.35 | - |
| 276Rg | 276.15849(67)# | 100# ms | Unknown | N/A | N/A | 1965.43 | - |
| 277Rg | 277.15952(66)# | 1# s | Unknown | N/A | N/A | 1973.51 | - |
| 278Rg | 278.16160(68)# | 1# s | a to 274Mt | N/A | N/A | 1972.27 | - |
| 279Rg | 279.16247(71)# | 0.17(+81-8) s | a to 275Mt | N/A | N/A | 1980.35 | - |
| 280Rg | 280.16447(80)# | 3.6(+43-13) s | a to 276Mt | N/A | N/A | 1988.43 | - |
| 281Rg | 281.16537(100)# | 1# min | SF; a to 277Mt | N/A | N/A | 1996.51 | - |
| 282Rg | 282.16749(95)# | 4# min | a to 278Mt | N/A | N/A | 2004.59 | - |
| 283Rg | 283.16842(84)# | 10# min | Unknown | N/A | N/A | 2012.66 | - |