About Oxides
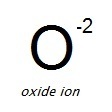
Oxides are compounds that contain one or more oxygen atoms and one or more other atoms. Oxide compounds make up a large proportion of the earth’s crust – nearly all metals naturally occur as minerals in oxide and compound forms which require processing and treatment to obtain the pure metal. The electronegativity of oxygen allows it to readily form compounds with almost all elements in nature, and most metals will spontaneously form oxides at their surface in oxygen-containing atmospheres.
#1: American Elements Develops Additives to Produce Ultra White Coating for Satellite Applications
Chemical Behavior and Classification
For many metals, oxidation can produce a uniform oxide coating termed a passivation layer. Once this coating has formed, it prevents further reaction of the underlying metal. Oxide passivation layers form spontaneously on some types of pure metals, such as aluminum or titanium, as well as on many corrosion-resistant alloys such as stainless steel. In other cases, chemical passivation processes such as chromate conversion coating are used to induce the formation of a protective oxide surface.
Most oxide compounds can also be classified as acidic, basic, or amphoteric based on the type of substances they react with. Typically, compounds of oxygen with nonmetals are acidic in aqueous solution, with the most electronegative elements producing the most acidic oxides. Binary compounds of oxygen with alkali, alkali earth, or transition metals produce basic solutions, with oxides of the more electropositive elements acting as the strongest bases. Finally, the post-transition metals and metalloids form oxides that may act as either acids or bases, and which are therefore considered amphoteric.
Many metal oxides have common names consisting of the metal element’s name modified with the suffix "-ia." Examples of this naming convention include alumina, titania, and zirconia.
Preparation of Metals from their Oxide Forms
The two most commonly used methods used to separate metals form their oxide forms are reduction and hydrolysis reactions. Reduction processes exploit redox chemistry and require a compound that more easily donates electrons than the element bonded to oxygen in the oxide. The most commonly used reducing agent in metallurgy is carbon in the form of coke, which can be used to win many metals from their oxide ores in what is termed a carbothermic reaction. Organic compounds are also sometimes used in reduction reactions to separate metals from their oxide forms. Hydrolysis is a method typically used with more reactive metal oxides, which can be dissolved into aqueous solution, sometimes with the assistance of a strong acid, and then recovered through precipitation steps or electrowinning processes.
Applications for Metal Oxides
The wide range of applications for metal oxides include their use as ceramics, refractory materials, pigments, lotions, coatings, magnetic applications, catalysis, and mesoporous materials. Ceramics often consist of metal oxides including zinc oxide, silicon dioxide, iron oxide, and aluminum oxide. Aluminum oxide-containing ceramics are commonly found in high temperature applications due to its high melting point. Refractory materials are both chemical and physically stable at high temperatures. For this reason use of these materials is common in engine components, high temperature furnaces, kilns, high friction applications, roofing, construction, and lighting. A common refractory medal oxide is zirconia, which can additionally be doped with other chemical elements including yttrium, scandium and cerium. Pigments are found in paints and cosmetics where titanium dioxide and zinc oxide compounds are typically found. Both zinc and titanium oxides are additional used in sunscreens and exterior surface coatings as UV protectants. Magnetic metal oxides are used in biomedical, magnetic data storage and electronics applications. For example, iron oxide nanoparticles are being studies for their efficacy in medical imaging and in antibody purification. Catalysis often requires metal oxides to improve reaction kinetics and provide either intermediate or final products. Areas of ongoing research in applying metal oxides to catalytic reactions are fuel and carbon dioxide (CO2) catalysis. In one Stanford University study, carbon dioxide reduction catalysis is achieved using copper oxide nanoparticles. Mesoporous materials have nanosize pores which are used in drug delivery, remediation, filtration, adsorption, and catalytic process applications. For example, water treatment frequently applies mesoporous transition metal oxides as catalysts. Common metal oxides used in mesoporous materials and in catalysis include titanium dioxide, iron oxide, zirconia, manganese oxide, and chromium oxide.
Products such as superconductors, semiconductor applications, optical windows, batteries, electronics and renewable energy products also include various metal oxides. Superconductors, for example, frequently use metal oxides and ceramics including copper oxide systems and iron oxide systems. Semiconductors such as MOSFETS (or metal oxide semiconductor field effect transistor) are transistors made of metal oxides used to amplify or toggle electronic signals. Photovoltaic or solar cells include multi-element oxides such as perovskite crystal-based systems can include aluminum oxide, titanium oxide, or zirconium oxide. Metal oxides can additionally be used as materials for solid oxide fuel cells (SOFCs), batteries, and advanced electronics.
Most Common Metallic Oxides in Commercial Applications
Numerous metal oxides occur in nature as ores and following purification for a given application are used across a wide range of industries including aerospace, biomedicine, automotive, construction, and consumer products. The following metal oxides are commonly found in commercial applications: aluminum oxide, titanium dioxide, zirconium oxide, zinc oxide, iron oxide, and silicon dioxide. Aluminum oxide exhibits high thermal conductivity, abrasiveness, and a high melting point. The compound is often found in paints, abrasive materials such as sandpaper, and as an ingredient in sunscreens, lipstick, and nail polish. Titanium dioxide is a bright white substance found in paints, creams, paper, plastic, coating of self-cleaning surfaces, and used as a disinfectant. Zirconia is used in ceramic knife blades such as ceramic kitchen knives, in metal forming tools, and as an additive to abrasive materials. Zinc oxide exhibits high thermal conductivity as well as antibacterial, and UV protective properties, lending it to applications including paints, surface protective coatings, lotions, powders, glazes, enamels, antibacterial substances, and food additives. Iron oxides are abundant compounds frequently used in coatings, paints, and as process catalysts. Silicon dioxide or silica exhibits electric insulation properties and is widely used for this purpose in microelectronics.
Below is only a limited selection of the full catalog of oxide products that American Elements manufactures. If you do not see a material you're looking for listed, please search the website or contact customerservice@americanelements.com.
 American Elements specializes in manufacturing single and multi-element oxides, doped oxides, and stabilized oxides in numerous different forms. Our standard products include powders and nanopowders, pieces, pellets, shot, tablets, and sputtering targets for thin film deposition, and forms for high temperature applications such as crucibles. We can also produce many forms not listed and custom compositions by request.
American Elements specializes in manufacturing single and multi-element oxides, doped oxides, and stabilized oxides in numerous different forms. Our standard products include powders and nanopowders, pieces, pellets, shot, tablets, and sputtering targets for thin film deposition, and forms for high temperature applications such as crucibles. We can also produce many forms not listed and custom compositions by request.