About Holmium
In 1878, Marc Delafontain and Jacques-Louis Soret identified spectroscopic absorption bands of an unknown element whose oxide was isolated later that same year by Per Teodor Cleve. All three men are given credit for discovering this new element, which was named holmium after Cleve’s native Stockholm.
Holmium has the greatest magnetic strength of any element, and is used to create extremely strong magnetic fields as a magnetic flux concentrator within high-strength magnets. Holmium is used as a magnet component instead of for fabricating complete magnets because it is extremely rare.
Holmium’s other primary use is as a dopant in garnets, cubic zirconia, and glass. Holmium garnets are used in solid-state lasers that emit at wavelengths that make them safe for use in medical and dental applications, and they are also used in fiber optic communications devices. In glass and cubic zirconia, holmium provides yellow or red coloring and has distinctive absorption peaks that make them useful as calibration standards for optical spectrophotometers. Additionally, the radioactive isotope holmium-166m1 is used to calibrate gamma ray spectrometers.
Holmium is a rare earth element and can be found in any rare earth containing mineral, but as a heavy rare earth element (HREE) it is more common in HREE-enriched minerals such as xenotime and euxenite. Additionally, holmium is present in ion adsorption clays, which are a major source of HREEs due to their relative ease of processing, despite the low percentage quantities of rare earths they contain.
Products
 Holmium has the highest magnetic moment (10.6µB) of any naturally occurring element. Because of this, it has been used to create the strongest known magnetic fields by placing it within high-strength magnets as a pole piece or magnetic flux concentrator. This magnetic property is also exploited in holmium-yttrium iron garnet, a synthetic ferrimagnetic material used in microwave and magnetooptical applications. Holmium lases at a human eye-safe 2.08 microns, allowing its use in a variety of medical and dental applications in both yttrium-aluminum-garnet (YAG) and yttrium-lanthanum-fluoride (YLF) solid state lasers. The wavelength
Holmium has the highest magnetic moment (10.6µB) of any naturally occurring element. Because of this, it has been used to create the strongest known magnetic fields by placing it within high-strength magnets as a pole piece or magnetic flux concentrator. This magnetic property is also exploited in holmium-yttrium iron garnet, a synthetic ferrimagnetic material used in microwave and magnetooptical applications. Holmium lases at a human eye-safe 2.08 microns, allowing its use in a variety of medical and dental applications in both yttrium-aluminum-garnet (YAG) and yttrium-lanthanum-fluoride (YLF) solid state lasers. The wavelength  allows for use in silica fibers designed for shorter wavelengths while still providing the cutting strength of longer wave length equipment. Holmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes.
allows for use in silica fibers designed for shorter wavelengths while still providing the cutting strength of longer wave length equipment. Holmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes.  Holmium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Holmium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Holmium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Holmium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Holmium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Holmium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Holmium Properties
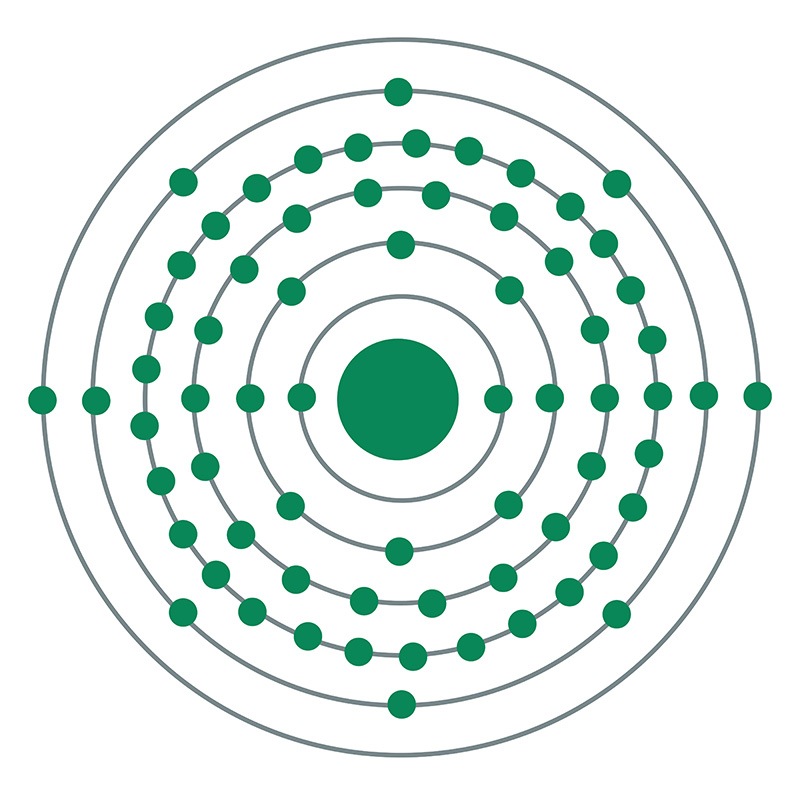
![]() Holmium is a Block F, Group 3, Period 6 element.
Holmium is a Block F, Group 3, Period 6 element.  The number of electrons in each of Holmium's shells is 2, 8, 18, 29, 8, 2 and its electronic configuration is [Xe] 4f11 6s2. In its elemental form, CAS 7440-60-0, holmium has a silvery white appearance. The holmium atom has a radius of 174.3.pm and its Van der Waals radius is unknown. Holmium was first discovered by J.L. Soret in 1878.
The number of electrons in each of Holmium's shells is 2, 8, 18, 29, 8, 2 and its electronic configuration is [Xe] 4f11 6s2. In its elemental form, CAS 7440-60-0, holmium has a silvery white appearance. The holmium atom has a radius of 174.3.pm and its Van der Waals radius is unknown. Holmium was first discovered by J.L. Soret in 1878.
Health, Safety & Transportation Information for Holmium
Holmium is slightly toxic in its elemental form. Safety data for Holmium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Holmium Isotopes
Holmium has one stable isotope: 165Ho.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 140Ho | 139.96854(54)# | 6(3) ms | Unknown | 8+# | N/A | 1093.11 | - |
| 141Ho | 140.96310(54)# | 4.1(3) ms | Unknown | (7/2-) | N/A | 1101.18 | - |
| 142Ho | 141.95977(54)# | 400(100) ms | ß+ to 142Dy; p to 141Dy | (6 to 9) | N/A | 1118.58 | - |
| 143Ho | 142.95461(43)# | 300# ms [>200 ns] | ß+ to 143Dy | 11/2-# | N/A | 1126.66 | - |
| 144Ho | 143.95148(32)# | 0.7(1) s | ß+ to 144Dy; ß+ + p to 143Tb | N/A | N/A | 1134.74 | - |
| 145Ho | 144.94720(32)# | 2.4(1) s | ß+ to 145Dy | (11/2-) | N/A | 1152.13 | - |
| 146Ho | 145.94464(21)# | 3.6(3) s | ß+ to 146Dy; ß+ + p to 145Tb | (10+) | N/A | 1160.21 | - |
| 147Ho | 146.94006(3) | 5.8(4) s | ß+ to 147Dy; ß+ + p to 146Tb | (11/2-) | N/A | 1168.29 | - |
| 148Ho | 147.93772(14) | 2.2(11) s | ß+ to 148Dy | (1+) | N/A | 1185.68 | - |
| 149Ho | 148.933775(20) | 21.1(2) s | ß+ to 149Dy | (11/2-) | N/A | 1193.76 | - |
| 150Ho | 149.933496(15) | 76.8(18) s | ß+ to 150Dy | 2- | N/A | 1201.84 | - |
| 151Ho | 150.931688(13) | 35.2(1) s | ß+ to 151Dy; a to 147Tb | 11/2(-) | N/A | 1209.92 | - |
| 152Ho | 151.931714(15) | 161.8(3) s | ß+ to 152Dy; a to 148Tb | 2- | N/A | 1218 | - |
| 153Ho | 152.930199(6) | 2.01(3) min | ß+ to 153Dy; a to 149Tb | 11/2- | N/A | 1226.08 | - |
| 154Ho | 153.930602(9) | 11.76(19) min | ß+ to 154Dy; a to 150Tb | 2- | N/A | 1234.16 | - |
| 155Ho | 154.929103(19) | 48(1) min | ß+ to 155Dy | 5/2+ | N/A | 1251.55 | - |
| 156Ho | 155.92984(5) | 56(1) min | ß+ to 156Dy | 4- | N/A | 1259.63 | - |
| 157Ho | 156.928256(26) | 12.6(2) min | ß+ to 157Dy | 7/2- | N/A | 1267.71 | - |
| 158Ho | 157.928941(29) | 11.3(4) min | ß+ to 158Dy; a to 154Tb | 5+ | N/A | 1275.79 | - |
| 159Ho | 158.927712(4) | 33.05(11) min | ß+ to 159Dy | 7/2- | N/A | 1283.87 | - |
| 160Ho | 159.928729(16) | 25.6(3) min | ß+ to 160Dy | 5+ | N/A | 1291.95 | - |
| 161Ho | 160.927855(3) | 2.48(5) h | EC to 161Dy | 7/2- | 4.25 | 1300.02 | - |
| 162Ho | 161.929096(4) | 15.0(10) min | EC to 161Dy | 1+ | N/A | 1308.1 | - |
| 163Ho | 162.9287339(27) | 4570(25) y | EC to 161Dy | 7/2- | 4.23 | 1316.18 | - |
| 164Ho | 163.9302335(30) | 29(1) min | EC to 161Dy; ß- to 164Er | 1+ | N/A | 1314.94 | - |
| 165Ho | 164.9303221(27) | Observationally Stable | - | 7/2- | 4.173 | 1323.02 | 100 |
| 166Ho | 165.9322842(27) | 26.83(2) h | ß- to 166Er | 0- | N/A | 1331.1 | - |
| 167Ho | 166.933133(6) | 3.003(18) h | ß- to 167Er | 7/2- | N/A | 1339.18 | - |
| 168Ho | 167.93552(3) | 2.99(7) min | ß- to 168Er | 3+ | N/A | 1347.26 | - |
| 169Ho | 168.936872(22) | 4.72(10) min | ß- to 169Er | 7/2- | N/A | 1355.34 | - |
| 170Ho | 169.93962(5) | 2.76(5) min | ß- to 170Er | 6+# | N/A | 1363.42 | - |
| 171Ho | 170.94147(64) | 53(2) s | ß- to 171Er | 7/2-# | N/A | 1362.18 | - |
| 172Ho | 171.94482(43)# | 25(3) s | ß- to 172Er | N/A | N/A | 1370.26 | - |
| 173Ho | 172.94729(43)# | 10# s | ß- to 173Er | 7/2-# | N/A | 1378.34 | - |
| 174Ho | 173.95115(54)# | 8# s | Unknown | N/A | N/A | 1377.1 | - |
| 175Ho | 174.95405(64)# | 5# s | Unknown | 7/2-# | N/A | 1385.18 | - |