About Gold
The notable properties of pure metallic gold are as follows: it is the most malleable and ductile of metals and an unusual color for elements of that class, is mostly non-reactive, conducts electricity well, and is extremely dense. The density of gold helped to drive its relative rarity, as gold present when the earth was formed would have largely sunk to the core of the planet. It is therefore believed that virtually all gold discovered by humans was deposited considerably later by meteorites containing the element. The low reactivity of gold explains why the metal was known to ancient societies despite its rarity: unlike most metals, it occurs mostly in its elemental form.
The rarity of gold, in combination with the ease with which it can be worked, its visual distinctiveness, and its resistance to chemical corrosion, made it an extremely unusual material and the object of much fascination. It was an obvious choice, then, for use as an ornamental status symbol and as a unit of monetary exchange. The oldest gold artifacts known have been dated to the 4th millennium BC, and the first gold coins (actually made of electrum, a natural gold-silver alloy) were minted around 600 BC in present-day Turkey. Gold remained a major component of monetary systems for much of the world into the twentieth century, as even when gold coinage became less common, most industrial economies used a gold standard to back their currencies. Gold standards started to be abandoned during World War I, and over time all modern industrialized nations switched to fiat currency systems.
Despite the fall of gold from an official monetary function, it is still widely viewed as valuable and used as an investment metal or means of storage of wealth, with many hoarding it as a hedge against inflation, and gold also remains a common metal used in fine jewelry. These functions still consume the majority of gold produced, despite a large number of other applications for the metal. Amalgams of gold and mercury have long been used in restorative dentistry for fillings and crowns, though concerns of mercury toxicity and the increasing availability of suitable composite materials as replacements have led to a decline in demand. Gold also finds many applications in electronics, where its high conductivity makes it attractive for wiring or as a coating for more easily corroded metals. Printed circuit boards often feature such thin protective gold layers. Thin films of gold are also useful for a variety of other functions. Gold can be manufactured to be thin enough to appear transparent, and thus be used in windows--in settings such as aircraft windshields--that can then be de-iced by passing electricity through the conductive film. Gold films are also excellent reflectors of electromagnetic radiation, including infrared light and radio waves, and are therefore used in infrared mirrors, heat shielding, and protective coatings on satellites and other equipment.
Despite being known for its low reactivity, it has long been known that gold can be dissolved in nitro-hydrochloric acid (aqua regia) and will form some compounds, including gold chlorides, gold oxides, and thiosulfates (such as gold sodium thiosulfate), and many applications of gold involve these less-familiar forms. Gold chloride solutions prepared by dissolving gold in aqua regia have been used to produce cranberry glass, the brilliant red color of which is now known to come from nanoscale gold particles dispersed within the glass.
Suspensions of such gold particles in liquid, also known as colloidal gold, are now of great interest due to their unique optical and electrical properties, in addition to their potential for useful interactions with biological systems. The electromagnetic absorption of colloidal gold solutions is tunable based on the size of the particles, a useful property with a side-effect of producing solutions that range in color from red to blue. Such solutions can be used in printable conductive inks for electronics, the production of sensors and photovoltaics, and the preparation of microscopy samples.
There is a significant history in modern biology of attaching tiny gold particles to a variety of biological probes, usually for use in electron microscopy, where the high electron-density of the gold particles makes them easy to visualize.Today, the ability to specifically target gold nanoparticles this way is being used in medicine, which prizes the ability to target specific tissues or cell types, including cancer cells. This allows them to be used in the detection of cancer cell locations and in the site-specific delivery of drugs and other therapeutic agents (including small RNA molecules under investigation for use as gene therapy). Additionally, gold nanorod structures absorb light in the near-infrared range, which easily passes through many human tissues. This fact has been exploited in cancer treatment: the heat generated when near-infrared light is absorbed by the rods kills the cells containing them, leaving surrounding cells largely unscathed.
Gold occurs most often as a native metal on its own or as natural gold-silver alloy. Most gold is mined in this form from either lode or placer deposits, and a small amount is produced as a byproduct of the processing of base metals. Additionally, gold is also frequently recycled from scrap, and many financial institutions still hold significant gold stockpiles.
Products
Gold is used in coinage and is a standard for most modern monetary systems. It is also used extensively for jewelry, dental restorations, and plating. It is used for coating certain space satellites, as it is inert and a good infrared reflector. The use of gold in electronics has seen significant growth, particularly within telecommunications, information technology and safety critical applications. Similarly, within computers there are usually gold-plated edge connectors. Gold bonding wires are used extensively within semiconductor packages and gold thick film inks are applied in the fabrication of hybrid circuits.  Gold's excellent solder wetting properties are used to form a very thin protective layer on copper laminate printed circuit boards. Gold is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).
Gold's excellent solder wetting properties are used to form a very thin protective layer on copper laminate printed circuit boards. Gold is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include pellets, rod, powder and granules for evaporation source material purposes. Gold nanoparticles and nanopowders are also available. Gold oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Gold is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, powder and granules for evaporation source material purposes. Gold nanoparticles and nanopowders are also available. Gold oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Gold is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
American Elements assists our Gold customers with fulfilling the due diligence reporting requirements of the Conflict Mineral Provision (Section 1502) of the Dodd-Frank Act.
Gold Properties
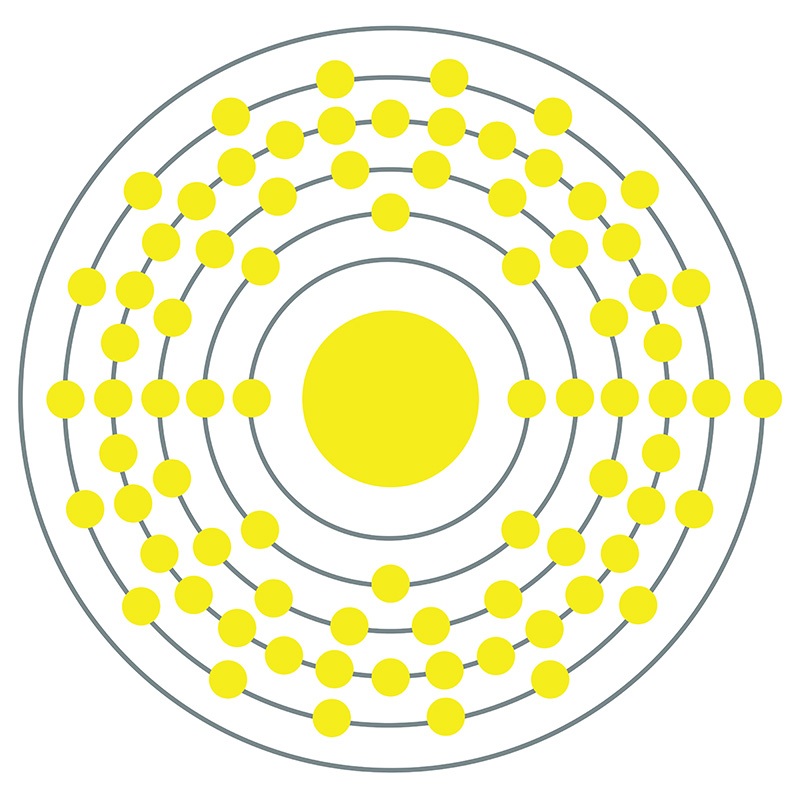
 Gold is a Block D, Group 11, Period 6 element. The number of electrons in each of Gold's shells is 2, 8, 18, 32, 18, 1 and its electronic configuration is [Xe] 4f142 5d10 6s1. The gold atom has a radius of 144.2.pm and its Van der Waals radius is 217.pm.
Gold is a Block D, Group 11, Period 6 element. The number of electrons in each of Gold's shells is 2, 8, 18, 32, 18, 1 and its electronic configuration is [Xe] 4f142 5d10 6s1. The gold atom has a radius of 144.2.pm and its Van der Waals radius is 217.pm.  In its elemental form, CAS 7440-57-5, gold has a metallic yellow appearance. Gold is a soft metal and is usually alloyed to give it more strength.
In its elemental form, CAS 7440-57-5, gold has a metallic yellow appearance. Gold is a soft metal and is usually alloyed to give it more strength. It is a good conductor of heat and electricity, and is unaffected by air and most reagents. It is one of the least reactive chemical elements. Gold is often found as a free element and with silver as a gold silver alloy. Less commonly, it is found in minerals as gold compounds, usually with tellurium. Gold was first discovered by early man c.a. 6000 BCE or earlier.
It is a good conductor of heat and electricity, and is unaffected by air and most reagents. It is one of the least reactive chemical elements. Gold is often found as a free element and with silver as a gold silver alloy. Less commonly, it is found in minerals as gold compounds, usually with tellurium. Gold was first discovered by early man c.a. 6000 BCE or earlier.
Health, Safety & Transportation Information for Gold
Gold is not toxic in its elemental form; however, safety data for gold and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab.
| Safety Data | |
|---|---|
| Signal Word | N/A |
| Hazard Statements | N/A |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | N/A |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
N/A |
Gold Isotopes
Gold (Au) has one stable isotope, 197Au
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 169Au | 168.99808(32)# | 150# µs | Unknown | 1/2+# | N/A | 1285.98 | - |
| 170Au | 169.99612(22)# | 310(50) µs [286(+50-40) µs] | Unknown | (2-) | N/A | 1294.05 | - |
| 171Au | 170.991879(28) | 17 µs | p to 170Pt; a to 167Ir | (1/2+) | N/A | 1302.13 | - |
| 172Au | 171.99004(17)# | 6.3 ms | a to 168Ir; p to 171Pt | high | N/A | 1310.21 | - |
| 173Au | 172.986237(28) | 20 ms | a to 169Ir; ß- to 173Pt | (1/2+) | N/A | 1327.61 | - |
| 174Au | 173.98476(11)# | 120 ms | a to 170Ir; ß- to 174Pt | low | N/A | 1335.68 | - |
| 175Au | 174.98127(5) | 185 ms | a to 171Ir; ß- to 175Pt | 1/2+# | N/A | 1343.76 | - |
| 176Au | 175.98010(11)# | 1.08(17) s [0.84(+17-14) s] | a to 172Ir; ß- to 176Pt | (5-) | N/A | 1351.84 | - |
| 177Au | 176.976865(14) | 1.462(32) s | ß- to 177Pt; a to 173Ir | (1/2+,3/2+) | N/A | 1369.24 | - |
| 178Au | 177.97603(6) | 2.6(5) s | ß- to 178Pt; a to 174Ir | N/A | N/A | 1377.32 | - |
| 179Au | 178.973213(18) | 3.3 s | ß- to 179Pt; a to 175Ir | 5/2-# | N/A | 1385.39 | - |
| 180Au | 179.972521(23) | 8.1(3) s | ß- to 180Pt; a to 176Ir | N/A | N/A | 1393.47 | - |
| 181Au | 180.970079(21) | 13.7(14) s | ß- to 181Pt; a to 177Ir | (3/2-) | N/A | 1401.55 | - |
| 182Au | 181.969618(22) | 15.5(5) s | ß- to 182Pt; a to 178Ir | (2+) | N/A | 1418.95 | - |
| 183Au | 182.967593(11) | 42.8(10) s | ß- to 183Pt; a to 179Ir | (5/2)- | N/A | 1427.03 | - |
| 184Au | 183.967452(24) | 20.6(9) s | ß- to 184Pt | 5+ | N/A | 1435.1 | - |
| 185Au | 184.965789(28) | 4.25(6) min | ß- to 185Pt; a to 180Ir | 5/2- | N/A | 1443.18 | - |
| 186Au | 185.965953(23) | 10.7(5) min | ß- to 186Pt; a to 181Ir | 3- | N/A | 1451.26 | - |
| 187Au | 186.964568(27) | 8.4(3) min | ß- to 187Pt; a to 182Ir | 1/2+ | N/A | 1459.34 | - |
| 188Au | 187.965324(22) | 8.84(6) min | ß- to 188Pt | 1(-) | N/A | 1467.42 | - |
| 189Au | 188.963948(22) | 28.7(3) min | ß- to 189Pt; a to 184Ir | 1/2+ | N/A | 1475.5 | - |
| 190Au | 189.964700(17) | 42.8(10) min | ß- to 190Pt; a to 185Ir | 1- | N/A | 1483.58 | - |
| 191Au | 190.96370(4) | 3.18(8) h | ß- to 191Pt | 3/2+ | N/A | 1491.66 | - |
| 192Au | 191.964813(17) | 4.94(9) h | ß- to 192Pt | 1- | N/A | 1499.73 | - |
| 193Au | 192.964150(11) | 17.65(15) h | ß- to 193Pt; a to 188Ir | 3/2+ | N/A | 1507.81 | - |
| 194Au | 193.965365(11) | 38.02(10) h | EC to 194Pt | 1- | 0.075 | 1515.89 | - |
| 195Au | 194.9650346(14) | 186.098(47) d | EC to 195Pt | 3/2+ | 0.149 | 1523.97 | - |
| 196Au | 195.966570(3) | 6.1669(6) d | EC to 196Pt; ß- to 196Hg | 2- | 0.591 | 1532.05 | - |
| 197Au | 196.9665687(6) | Observationally Stable | - | 3/2+ | 0.148159 | 1540.13 | 100 |
| 198Au | 197.9682423(6) | 2.69517(21) d | ß- to 198Hg | 2- | 0.5934 | 1548.21 | - |
| 199Au | 198.9687652(6) | 3.139(7) d | ß- to 199Hg | 3/2+ | 0.2715 | 1556.28 | - |
| 200Au | 199.97073(5) | 48.4(3) min | ß- to 200Hg | 1(-) | N/A | 1555.05 | - |
| 201Au | 200.971657(3) | 26(1) min | ß- to 201Hg | 3/2+ | N/A | 1563.13 | - |
| 202Au | 201.97381(18) | 28.8(19) s | ß- to 202Hg | (1-) | N/A | 1571.2 | - |
| 203Au | 202.975155(3) | 53(2) s | ß- to 203Hg | 3/2+ | N/A | 1579.28 | - |
| 204Au | 203.97772(22)# | 39.8(9) s | ß- to 204Hg | (2-) | N/A | 1587.36 | - |
| 205Au | 204.97987(32)# | 31(2) s | ß- to 205Hg | 3/2+ | N/A | 1595.44 | - |