SECTION 1. IDENTIFICATION
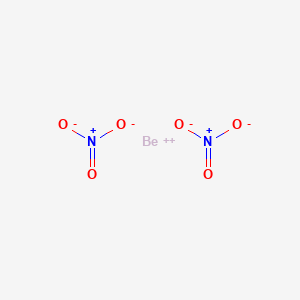
Product Name: Beryllium Nitrate
Product Number: All applicable American Elements product codes, e.g. BE-NAT-02
, BE-NAT-03
, BE-NAT-04
, BE-NAT-05
CAS #: 13597-99-4
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Physical hazards Oxidizing solids Category 2
Health hazards Acute toxicity, oral Category 3
Acute toxicity, inhalation Category 2
Skin corrosion/irritation Category 2
Serious eye damage/eye irritation Category 2
Sensitization, skin Category 1
Carcinogenicity (inhalation) Category 1B
Specific target organ toxicity, single exposure Category 3 respiratory tract irritation
Specific target organ toxicity, repeated Category 1 (Respiratory system)
exposure
Environmental hazards Not classified.
OSHA defined hazards Not classified.
Label elements
Signal word: Danger
Hazard statement In contact with water releases flammable gas. Toxic if swallowed. Fatal if inhaled. Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. May cause cancer by inhalation. May cause respiratory irritation. Causes damage to organs (respiratory system) through prolonged or repeated exposure by inhalation.
Precautionary statement
Prevention Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Minimize dust generation and accumulation. Avoid breathing dust. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. Contaminated work clothing must not be allowed out of the workplace. Wear protective gloves/protective clothing/eye protection/face protection. In case of inadequate ventilation wear respiratory protection.
Response If swallowed: Immediately call a poison center/doctor. If on skin: Wash with plenty of water. If inhaled: Remove person to fresh air and keep comfortable for breathing. If in eyes: Rinse
cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing. If exposed or concerned: Get medical advice/attention. If skin irritation or rash
occurs: Get medical advice/attention. Wash contaminated clothing before reuse. If experiencing
respiratory symptoms: Call a poison center/doctor.
Storage Store locked up. Store in a well-ventilated place. Keep container tightly closed.
Disposal Dispose of contents/container in accordance with local/regional/national/international regulations.
Hazard(s) not otherwise
classified (HNOC)
None known.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Beryllium Nitrate
CAS number: 13597-99-4
SECTION 4. FIRST AID MEASURES
Inhalation
If symptoms develop move victim to fresh air. For breathing difficulties, oxygen may be necessary.
Breathing difficulty caused by inhalation of particulate requires immediate removal to fresh air. If
breathing has stopped, perform artificial respiration and obtain medical help.
Skin contact
Take off contaminated clothing and wash before reuse. Thoroughly wash skin cuts or wounds to
remove all particulate debris from the wound. Seek medical attention for wounds that cannot be
thoroughly cleansed. Treat skin cuts and wounds with standard first aid practices such as
cleansing, disinfecting and covering to prevent wound infection and contamination before
continuing work. Obtain medical help for persistent irritation. Material accidentally implanted or
lodged under the skin must be removed.
Eye contact
Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids
occasionally. Get medical attention if symptoms persist.
Ingestion
If swallowed, seek medical advice immediately and show this container or label. Induce vomiting
immediately as directed by medical personnel. Never give anything by mouth to an unconscious
person.
Most important symptoms/effects, acute and delayed
May cause allergic skin reaction. May cause allergic respiratory reaction. Prolonged exposure may
cause chronic effects.
Indication of immediate medical attention and special treatment needed
Treatment of Chronic Beryllium Disease: There is no known treatment which will cure chronic
beryllium disease. Prednisone or other corticosteroids are the most specific treatment currently
available. They are directed at suppressing the immunological reaction and can be effective in
diminishing signs and symptoms of chronic beryllium disease. In cases where steroid therapy has had only partial or minimal effectiveness, other immunosuppressive agents, such as
cyclophosphamide, cyclosporine, or methotrexate, have been used. In view of the potential side
effects of all the immunosuppressive medications, including steroids such as prednisone, they
should be used only under the direct care of a physician. Other treatment, such as oxygen, inhaled
steroids or bronchodilators, may be prescribed by some physicians and can be effective in
selected cases. In general, treatment is reserved for cases with significant symptoms and/or
significant loss of lung function. The decision about when and with what medication to treat is a
judgment situation for individual physicians.
SECTION 5. FIREFIGHTING MEASURES
Suitable extinguishing media
The product is non-combustible. Use extinguishing measures that are appropriate to local
circumstances and the surrounding environment.
Special protective equipment and precautions for firefighters
Firefighters should wear full protective clothing including self contained breathing apparatus. Wear
suitable protective equipment.
Fire fighting equipment/instructions
Move containers from fire area if you can do so without risk. Water runoff can cause environmental
damage. Do not use water to extinguish fires around operations involving molten metal due to the
potential for steam explosions.
Specific methods
Pressure-demand self-contained breathing apparatus must be worn by firefighters or any other
persons potentially exposed to the particulate released during or after a fire.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
In solid form this material poses no special clean-up problems. Wear appropriate protective
equipment and clothing during clean-up.
Methods and materials for containment and cleaning up
Clean up in accordance with all applicable regulations.
Environmental precautions
Avoid release to the environment. In the event of a spill or accidental release, notify relevant
authorities in accordance with all applicable regulations. Prevent further leakage or spillage if safe
to do so. Avoid discharge into drains, water courses or onto the ground.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling
Obtain special instructions before use. Do not handle until all safety precautions have been read
and understood. Minimize dust generation and accumulation. Do not breathe dust/fume. Wear
protective gloves/protective clothing/eye protection/face protection. Wear respiratory protection.
Wash thoroughly after handling. When using, do not eat, drink or smoke. Contaminated work
clothing must not be allowed out of the workplace.
Conditions for safe storage, including any incompatibilities
Keep locked-up. Avoid contact with acids and alkalies. Avoid contact with oxidizing agents.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Exposure guidelines
On July 14, 2020, the Occupational Safety and Health Administration (OSHA) issued the final
Beryllium Standard for General Industry (29 CFR 1910.1024) which includes a Permissible
Exposure Limit (PEL) of 0.2 μg/m3 as an 8-hour TWA. The Preamble to the OSHA Beryllium
Standards in 29 CFR Parts 1910, 1915 and 1926 states: “OSHA concludes that exposure to
beryllium constitutes a significant risk of material impairment to health and that the final rule will
substantially lower that risk. The Agency considers the level of risk remaining at the new TWA PEL
to still be significant. However, OSHA did not adopt a lower TWA PEL because the Agency could
not demonstrate technological feasibility of a lower TWA PEL. The Agency has adopted the STEL
and ancillary provisions of the rule to further reduce the remaining significant risk.”
Based on joint research conducted with the National Institute for Occupational Safety and Health
(NIOSH), Materion adopted an 8 element Beryllium Worker Protection Model (BWPM) which
includes the use of a recommended exposure guideline (REG) for airborne beryllium of 0.2 μg/m3
as a time-weighted average (TWA) limit for an 8-hour work day. Subsequent NIOSH studies have
shown that the BWPM has reduced but not eliminated the risk of beryllium sensitization and
chronic beryllium disease (CBD) in workers. Therefore, Materion recommends that beryllium users
not only comply with the OSHA Beryllium Standard and carefully apply all elements of the BWPM,
but reduce airborne exposures to the lowest feasible level. Information on the BWPM can be
found at www.berylliumsafety.com or by contacting Materion at +1 800.862.4118.
The American Conference of Governmental Industrial Hygienists (ACGIH®) is a scientific body
that has developed guidelines for all listed substances. In its development documents, the
ACGIH® states that “Threshold Limit Values and Biological Exposure Indices represent conditions
under which ACGIH® believes that nearly all workers may be repeatedly exposed without adverse
health effects. They are not fine lines between safe and dangerous exposures, nor are they a
relative index of toxicology.”
Specific genetic factors have been identified and shown to increase an individual’s susceptibility to
CBD. Medical testing is available to detect those genetic factors in individuals.
Appropriate engineering controls
Ensure adequate ventilation, especially in confined areas.
Good general ventilation (typically 10 air changes per hour) should be used. Ventilation rates
should be matched to conditions. If applicable, use process enclosures, local exhaust ventilation,
or other engineering controls to maintain airborne levels below recommended exposure limits. If
exposure limits have not been established, maintain airborne levels to an acceptable level.
Whenever possible, the use of local exhaust ventilation or other engineering controls is the
preferred method of controlling exposure to airborne particulate. Where utilized, exhaust inlets to
the ventilation system must be positioned as close as possible to the source of airborne
generation. Avoid disruption of the airflow in the area of a local exhaust inlet by equipment such as
a man-cooling fan. Check ventilation equipment regularly to ensure it is functioning properly.
Provide training on the use and operation of ventilation to all users. Use qualified professionals to
design and install ventilation systems.
Eye/face protection
Wear approved safety glasses, goggles, face shield and/or welder’s helmet when risk of eye injury
is present, particularly during operations that generate dust, mist or fume.
Hand protection
Wear gloves to prevent contact with particulate or solutions. Wear gloves to prevent metal cuts
and skin abrasions during handling.
Respiratory protection
When airborne exposures exceed or have the potential to exceed the occupational exposure
limits, approved respirators must be used as specified by an Industrial Hygienist or other qualified
professional. Respirator users must be medically evaluated to determine if they are physically
capable of wearing a respirator. Quantitative and/or qualitative fit testing and respirator training
must be satisfactorily completed by all personnel prior to respirator use. Users of tight fitting
respirators must be clean shaven on those areas of the face where the respirator seal contacts the
face. Use pressure-demand airline respirators when performing jobs with high potential exposures such as changing filters in a baghouse air cleaning device.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance
Physical state Solid.
Form Crystalline.
Color White. Yellow.
Odor Nitrogen Pentoxide Odor.
Odor threshold Not applicable.
pH Not applicable.
Melting point/freezing point 140.9 °F (60.5 °C) / Not applicable.
Initial boiling point and boiling range
Not applicable.
Flash point Not applicable.
Evaporation rate Not applicable.
Flammability (solid, gas) Flammable gas.
Upper/lower flammability or explosive limits
Explosive limit - lower (%) Not applicable.
Explosive limit - upper (%) Not applicable.
Vapor pressure Not applicable.
Vapor density Not applicable.
Relative density Not applicable.
Solubility(ies)
Solubility (water) Soluble.
Partition coefficient
(n-octanol/water)
Not applicable.
Auto-ignition temperature Not applicable.
Decomposition temperature Not applicable.
Viscosity Not applicable.
Other information
Density Not available.
Molecular formula Be.2H-N-O3
Molecular weight 133.03 g/mol
SECTION 10. STABILITY AND REACTIVITY
Reactivity Not available.
Chemical stability Not available.
Possibility of hazardous Not available.
reactions
Conditions to avoid Not available.
Incompatible materials Not available.
Hazardous decomposition products Not available.
SECTION 11. TOXICOLOGICAL INFORMATION
Information on likely routes of exposure
Inhalation May cause sensitization by inhalation. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause damage to organs (respiratory system) through prolonged or repeated exposure.
Skin contact May cause an allergic skin reaction.
Eye contact Not likely, due to the form of the product.
Ingestion Not likely, due to the form of the product.
Symptoms related to the physical, chemical and toxicological characteristics
Respiratory disorder.
Information on toxicological effects
Acute toxicity May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause allergic skin reaction.
Skin corrosion/irritation Not likely, due to the form of the product.
Serious eye damage/eye Harmful in contact with eyes.
irritation
Respiratory or skin sensitization
ACGIH sensitization
BERYLLIUM AND COMPOUNDS, SOLUBLE AND
INSOLUBLE COMPOUNDS, AS BE, INHALABLE
FRACTION (CAS 13597-99-4)
Respiratory sensitization
Respiratory sensitization May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Skin sensitization May cause an allergic skin reaction.
Germ cell mutagenicity Due to lack of data the classification is not possible.
Carcinogenicity Cancer hazard.
IARC Monographs. Overall Evaluation of Carcinogenicity
Beryllium Nitrate (CAS 13597-99-4) 1 Carcinogenic to humans.
OSHA Specifically Regulated Substances (29 CFR 1910.1001-1053)
Beryllium Nitrate (CAS 13597-99-4) Cancer
US. National Toxicology Program (NTP) Report on Carcinogens
Beryllium Nitrate (CAS 13597-99-4) Known To Be Human Carcinogen.
Reproductive toxicity Not classified.
Specific target organ toxicity -single exposure
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Specific target organ toxicity -repeated exposure
May cause damage to organs (respiratory system) through prolonged or repeated exposure by
inhalation.
Chronic effects Hazardous by OSHA criteria. May cause damage to organs through prolonged or repeated exposure.
Further information Symptoms may be delayed.
SECTION 12. ECOLOGICAL INFORMATION
Persistence and degradability No data is available on the degradability of this product.
Bioaccumulative potential Not available.
Mobility in soil Not available.
Other adverse effects Not available.
SECTION 13. DISPOSAL CONSIDERATIONS
Disposal instructions
Material should be recycled if possible. Disposal recommendations are based on material as
supplied. Disposal must be in accordance with current applicable laws and regulations, and
material characteristics at time of disposal. When this product as supplied is to be discarded as
waste, it does not meet the definition of a RCRA waste under 40 CFR 261.
Waste from residues / unused products
Empty containers or liners may retain some product residues. This material and its container must
be disposed of in a safe manner (see: Disposal instructions).
Contaminated packaging
Empty containers should be taken to an approved waste handling site for recycling or disposal.
Since emptied containers may retain product residue, follow label warnings even after container is
emptied.
SECTION 14. TRANSPORT INFORMATION
DOT
UN number UN2464
UN proper shipping name Beryllium nitrate
Class 5.1
Transport hazard class(es)
Subsidiary risk 6.1(PGI, II)
Label(s) 5.1, 6.1
Packing group II
Special precautions for user Not available.
Special provisions IB8, IP2, IP4, T3, TP33
Packaging exceptions 152
Packaging non bulk 212
Packaging bulk 242
IATA
UN number UN2464
UN proper shipping name Beryllium nitrate
Class 5.1
Transport hazard class(es)
Subsidiary risk 6.1(PGI, II)
Packing group II
Environmental hazards No.
ERG Code 5P
Special precautions for user Not available.
Other information
Cargo aircraft only Allowed with restrictions.
IMDG
UN number UN2464
UN proper shipping name BERYLLIUM NITRATE
Class 5.1
Transport hazard class(es)
Subsidiary risk 6.1(PGI, II)
Packing group II
Marine pollutant No.
Environmental hazards
EmS F-A, S-Q
Special precautions for user Not available.
SECTION 15. REGULATORY INFORMATION
US federal regulations This product is listed on the U.S. EPA TSCA Inventory.
Toxic Substances Control Act (TSCA)
TSCA Section 12(b) Export Notification (40 CFR 707, Subpt. D)
Not regulated.
CERCLA Hazardous Substance List (40 CFR 302.4)
Beryllium Nitrate (CAS 13597-99-4) Listed.
SARA 304 Emergency release notification
Not regulated.
OSHA Specifically Regulated Substances (29 CFR 1910.1001-1053)
Beryllium Nitrate (CAS 13597-99-4) Cancer
lung effects (CBD and acute beryllium disease)
beryllium sensitization
respiratory tract irritation
SARA 302 Extremely hazardous substance
Superfund Amendments and Reauthorization Act of 1986 (SARA)
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.

 The number of electrons in each of Beryllium's shells is [2, 2] and its electron configuration is [He] 2s2. The beryllium atom has a radius of 112 pm and a Van der Waals radius of 153 pm. Beryllium is a relatively rare element in the earth's crust; it can be found in minerals such as bertrandite, chrysoberyl, phenakite, and beryl, its most common source for commercial production. Beryllium was discovered by Louis Nicolas Vauquelin in 1797 and first isolated by Friedrich Wöhler and Antoine Bussy in 1828.
The number of electrons in each of Beryllium's shells is [2, 2] and its electron configuration is [He] 2s2. The beryllium atom has a radius of 112 pm and a Van der Waals radius of 153 pm. Beryllium is a relatively rare element in the earth's crust; it can be found in minerals such as bertrandite, chrysoberyl, phenakite, and beryl, its most common source for commercial production. Beryllium was discovered by Louis Nicolas Vauquelin in 1797 and first isolated by Friedrich Wöhler and Antoine Bussy in 1828.  In its elemental form, beryllium has a gray metallic appearance. It is a soft
In its elemental form, beryllium has a gray metallic appearance. It is a soft