About Beryllium
Beryllium-containing minerals have been known by humans since at least the Ptolemaic dynasty of Egypt, where some were recognized as gemstones. The first chemical analysis that succeeding in distinguishing a common beryllium mineral, beryl, from other aluminum silicates was completed in 1789 by the chemist Louis Vauquelin, to whom credit is given for the discovery of this element. Editors of the journal in which he published his findings favored the name “glucine” for the element due to the sweet taste of some of its compounds. However, the name suggested by Friedrich Wohler, who became the first to isolate pure metallic beryllium in 1828, was the one that ultimately became official.
Beryllium is an extremely lightweight metal that is brittle at room temperature but also extremely hard. Occupying the fourth spot on the periodic table, beryllium heads the alkaline earth group of metals that includes calcium and magnesium and is the lightest of all metals except for lithium. High thermal conductivity, heat capacity, and melting point (the highest of the so-called “light metals”) make beryllium a useful industrial metal, especially because its alloys are tough, stiff, and lightweight. Beryllium-copper in particular (also known as beryllium bronze) conducts electricity and heat almost as well as pure copper but is harder, stronger, and more corrosion-resistant than other copper-based alloys. Beryllium-copper has been used in multiple capacities ranging from electrical contacts in high-performance circuit boards, automobile and aircraft parts, oil and gas drilling equipment, and radar systems. It also has the unusual ability to be struck without giving off sparks, making it useful in tools and equipment in high-temperature, explosive environments such as rocket manufacturing facilities. Other alloys include nickel-beryllium, a high-strength precipitation hardenable alloy, and aluminum-beryllium, three times as stiff and twenty-five times lighter than aluminum, used in helicopters and satellite guidance systems.
Outside of use as an alloying agent, beryllium is primarily valued for its nuclear and electronic properties. Due to its low atomic mass and density, beryllium is largely transparent to X-rays and remains stable under neutron bombardment. It is frequently used as a window or coating for x-ray tubes, and ultrathin beryllium foil is used in x-ray lithography. It also has various functions in the production of nuclear fuels and the construction of nuclear reactors. In electronic devices, beryllium serves as a p-type dopant in III-V compound semiconductors, and can also be used as both a structural support and heat sink for printed circuit boards. Frequently, a composite of beryllium and beryllium oxide is used for this purpose.
Beryllium oxide is a particularly notable as a compound as it is both an electrical insulator and a good thermal conductor, and additionally exhibits strength, hardness, and a high melting point. These properties lend it to a variety of uses as a refractory ceramic primarily as sintered crucibles, bricks, and heat sinks in many electronics.
Beryllium and its compounds are toxic when ingested, as the element can substitute for its neighbor on the periodic table, magnesium, in essential enzymes, causing dysfunction. However, beryllium is poorly absorbed through the skin and the digestive tract, so beryllium poisoning by those routes is rare. The largest real safety concern with beryllium is inhalation of dust containing the element. The dust is an irritant, and can cause either acute lung ailments or a chronic lung disease called berylliosis, depending on the dose and time course of exposure. Acute overexposure is relatively rare, but chronic beryllium disease is a major health concern, as even very low levels can cause significant harm after years of exposure. Current guidelines suggest keeping airborne beryllium exposure to “the lowest feasible level” at all times. Beryllium was once widely used in the production of fluorescent lighting, but this use has been discontinued as the exposure of workers to beryllium was too high.
Beryllium is not found in large quantities as a free element, but is a component of many naturally occurring minerals. These minerals occur only rarely in significant exploitable deposits, and both this and the expense of extracting the element from ore contribute to its high cost. The primary ores for the element are bertrandite, which is a beryllium sorosilicate hydroxide mineral, and beryl, an aluminum beryllium cyclosilicate. Some high-quality samples of beryl that contain impurities that produce attractive colors are considered precious stones; these include aquamarine, red beryl, and emerald.
Beryllium’s high affinity for oxygen at high temperatures, along with its ability to reduce water, make it difficult to extract from its mineral forms. There are two processes currently in use to accomplish this: in the melt method, beryl is ground into a powder and heated to 3000 °F. It is then cooled and reheated with acid, producing beryllium sulfate, and treated with ammonia to produce beryllium hydroxide. In the sintering method, beryl is sintered at 1420F with sodium fluorosilicate and soda, producing sodium fluoroberyllate. This product is water soluble, and adding sodium hydroxide to a solution of fluoroberyllate produces beryllium hydroxide. Beryllium hydroxide obtained by either method can be converted to either beryllium fluoride or beryllium chloride, which may be used directly or converted to pure beryllium metal.
Due to the complexity of this process, recycling beryllium alloys and scrap is substantially more energy efficient than producing new metal from ore. It is estimated that currently 20-25% of beryllium used annually is recycled, and the introduction of new recycling programs in recent years suggests that this number may rise in the near future.
Products
Beryllium is a fairly soft metal that is brittle yet strong. It is used as a coating on X-ray tubes because it is transparent to the X-ray range. It also has military and nuclear industry applications.  Beryllium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).
Beryllium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Beryllium oxide is an insoluble source of beryllium available in powder and dense pellet form for such uses as optical coating and thin film applications. Beryllium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Beryllium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Beryllium oxide is an insoluble source of beryllium available in powder and dense pellet form for such uses as optical coating and thin film applications. Beryllium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Beryllium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Beryllium Properties
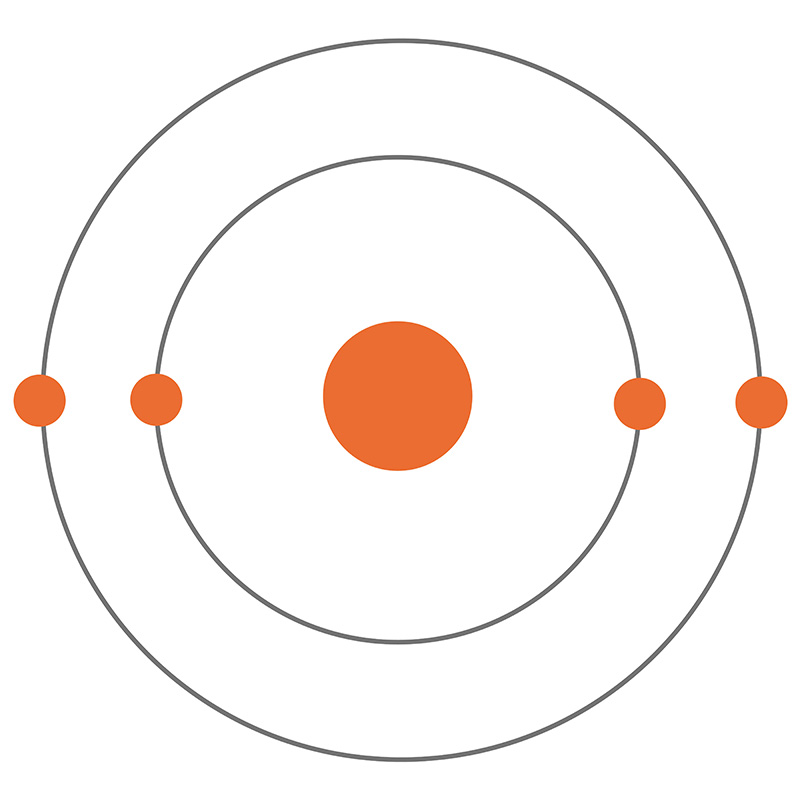
![]() Beryllium is a Block S, Group 2, Period 2 element. The number of electrons in each of Beryllium's shells is 2, 2 and its electron configuration is [He] 2s2.
Beryllium is a Block S, Group 2, Period 2 element. The number of electrons in each of Beryllium's shells is 2, 2 and its electron configuration is [He] 2s2.  The most common source for commercial production of Beryllium is beryl, a barium aluminosilicate mineral. Beryllium is also found in bertrandite (Be4Si2O7(OH)2), chrysoberyl (Al2BeO4) and phenakite (Be2SiO4). Beryllium was discovered by Louis Nicolas Vauquelin in 1797. It was first isolated by Friedrich Wöhler & Antoine Bussy in 1828. The origin of the name Beryllium comes from the Greek word 'beryllos' meaning beryl.
The most common source for commercial production of Beryllium is beryl, a barium aluminosilicate mineral. Beryllium is also found in bertrandite (Be4Si2O7(OH)2), chrysoberyl (Al2BeO4) and phenakite (Be2SiO4). Beryllium was discovered by Louis Nicolas Vauquelin in 1797. It was first isolated by Friedrich Wöhler & Antoine Bussy in 1828. The origin of the name Beryllium comes from the Greek word 'beryllos' meaning beryl.
Health, Safety & Transportation Information for Beryllium
Beryllium and its salts are toxic as well as carcinogenic. Safety data for Beryllium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H317-H319-H330-H335-H350i-H372 |
| Hazard Codes | T+ |
| Risk Codes | 49-25-26-36/37/38-43-48/23 |
| Safety Precautions | 53-45 |
| RTECS Number | DS1750000 |
| Transport Information | UN 1567 6.1/PG 2 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Beryllium Isotopes
Beryllium has one stable isotope: 9Be.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 5Be | 5.04079(429)# | N/A | p to 4Li | (1/2+)# | N/A | -2.01 | - |
| 6Be | 6.019726(6) | 5.0(3)E-21 s [0.092(6) ] | 2p to 4He | 0+ | N/A | 25.63 | - |
| 7Be | 7.01692983(11) | 53.22(6) d | EC to 7Li | 3/2- | N/A | 36.32 | - |
| 8Be | 8.00530510(4) | 6.7(17)E-17 s [6.8(17) eV] | 2a to n | 0+ | N/A | 55.2 | - |
| 9Be | 9.0121822(4) | STABLE | - | 3/2- | -1.1779 | 56.95 | 100 |
| 10Be | 10.0135338(4) | 1.51(6)x106 y | ß- to 10B | 0+ | N/A | 64.19 | - |
| 11Be | 11.021658(7) | 13.81(8) s | ß- to 11B; ß- + a to 7Li | 1/2+ | N/A | 64.81 | - |
| 12Be | 12.026921(16) | 21.49(3) ms | ß- to 12B; ß- + n to 11B | 0+ | N/A | 68.23 | - |
| 13Be | 13.03569(8) | .5(1) ns | n to 12Be | 1/2+ | N/A | 67.93 | - |
| 14Be | 14.04289(14) | 4.84(10) ms | ß- + n to 13B; ß- to 14B; ß- + 2n to 12B | 0+ | N/A | 69.49 | - |
| 15Be | 15.05346(54)# | <200 ns | Unknown | N/A | N/A | 67.32 | - |
| 16Be | 16.06192(54)# | <200 ns | 2n to 14Be | 0+ | N/A | 67.94 | - |