SECTION 1. IDENTIFICATION
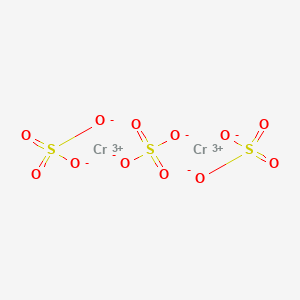
Product Name: Chromium Sulfate
Product Number: All applicable American Elements product codes, e.g. CR-SAT-02
, CR-SAT-03
, CR-SAT-04
, CR-SAT-05
CAS #: 10101-53-8
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Appearance: green to violet to red solid.
Warning! Causes eye, skin, and respiratory tract irritation. May cause allergic skin reaction.
Target Organs: Respiratory system, eyes, skin.
Potential Health Effects
Eye: Causes eye irritation.
Skin: Causes skin irritation. May cause skin sensitization, an allergic reaction, which becomes
evident upon re-exposure to this material.
Ingestion: Causes gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation: Causes respiratory tract irritation.
Chronic: No information found.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
CAS# Chemical Name Percent EINECS/ELINCS
10101-53-8 Chromium (III) Sulfate Hydrate 100% 233-253-2
SECTION 4. FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and
lower eyelids. If irritation develops, get medi cal aid.
Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing
contaminated clothing and shoes.
Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid.
Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give
artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician: Treat symptomatically and supportively.
SECTION 5. FIREFIGHTING MEASURES
General Information: As in any fire, wear a self-contained breathing apparatus in pressuredemand,
MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating
and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from
fire control or dilution water may cause pollution.
Extinguishing Media: Substance is noncombustible; use agent most appropriate to extinguish
surrounding fire.
Flash Point: Not available.
Autoignition Temperature: Not available.
Explosion Limits, Lower:Not available.
Upper: Not available.
NFPA Rating: (estimated) Health: 1; Flammability: 0; Instability: 0
SECTION 6. ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Clean
up spills immediately, observing precautions in the Protective Equipment section. Avoid
generating dusty conditions. Provide ventilation.
SECTION 7. HANDLING AND STORAGE
Handling: Minimize dust generation and accumulation. Avoid contact with skin and eyes. Keep
container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage: Store in a cool, dry place. Store in a tightly closed container.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Engineering Controls: Facilities storing or utilizing this material should be equipped with an
eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep
airborne concentrations below the permissible exposure limits.
Exposure Limits
Chemical Name ACGIH NIOSH OSHA - Final PELs
Chromium (III) Sulfate
Hydrate none listed none listed none listed
OSHA Vacated PELs: Chromium (III) Sulfate Hydrate: No OSHA Vacated PELs are listed for this
chemical.
Personal Protective Equipment
Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by
OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin: Wear appropriate protective gloves to prevent skin exposure.
Clothing: Wear appropriate protective clothing to minimize contact with skin.
Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European
Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if
exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Appearance: green to violet to red
Odor: odorless
pH: 1.0-2.5 5% solution
Vapor Pressure: Negligible.
Vapor Density: Not available.
Evaporation Rate:N/A
Viscosity: N/A
Boiling Point: Not available.
Freezing/Melting Point:Not available.
Decomposition Temperature:Not available.
Solubility: Soluble in water.
Specific Gravity/Density:1.7-3.0
Molecular Formula:Cr2(SO4)3.nH2O
Molecular Weight:392.1648
SECTION 10. STABILITY AND REACTIVITY
Chemical Stability: Stable under normal temperatures and pressures.
Conditions to Avoid: None reported.
Incompatibilities with Other Materials: Hydrogen gas may be evolved from moist chromic
sulfate. If damp material is sealed for a prolonged period of time, the container may rupture
because of the pressure of hydrogen. Reacts violently with reducing agents, combustibles,
ammonia, halides, phosphorous, sodium azide, elemental sulfur and urea.
Hazardous Decomposition Products: Oxides of sulfur, irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
SECTION 11. TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 10101-53-8: GB7200000
LD50/LC50:
Not available.
Carcinogenicity:
CAS# 10101-53-8: Not listed by ACGIH, IARC, NTP, or CA Prop 65.
Epidemiology: No information found
Teratogenicity: No information found
Reproductive Effects: No information found
Mutagenicity: Mutagenic effects have occurred in humans.
Neurotoxicity: No information found
Other Studies:
SECTION 12. ECOLOGICAL INFORMATION
No data available.
SECTION 13. DISPOSAL CONSIDERATIONS
Chemical waste generators must determine whether a discarded chemical is classified as a
hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR Parts
261.3. Additionally, waste generators must consult state and local hazardous waste regulations
to ensure complete and accurate classification.
RCRA P-Series: None listed.
RCRA U-Series: None listed.
SECTION 14. TRANSPORT INFORMATION
US DOT Canada TDG
Shipping Name: Not regulated as a hazardous material No data available.
Hazard Class:
UN Number:
Packing Group:
SECTION 15. REGULATORY INFORMATION
US FEDERAL
TSCA
CAS# 10101-53-8 is listed on the TSCA inventory.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
CERCLA Hazardous Substances and corresponding RQs
CAS# 10101-53-8: 1000 lb final RQ; 454 kg final RQ
SARA Section 302 Extremely Hazardous Substances
None of the chemicals in this product have a TPQ.
SARA Codes
CAS # 10101-53-8: immediate, delayed.
Section 313 No chemicals are reportable under Section 313.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
This material does not contain any Class 2 Ozone depletors.
Clean Water Act:
CAS# 10101-53-8 is listed as a Hazardous Substance under the CWA.
None of the chemicals in this product are listed as Priority Pollutants under the CWA.
None of the chemicals in this product are listed as Toxic Pollutants under the CWA.
OSHA:
None of the chemicals in this product are considered highly hazardous by OSHA.
STATE
CAS# 10101-53-8 can be found on the following state right to know lists: California, New
Jersey, Pennsylvania, Massachusetts.
California Prop 65
California No Significant Risk Level: None of the chemicals in this product are listed.
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols:
XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 10101-53-8: 1
Canada - DSL/NDSL
CAS# 10101-53-8 is listed on Canada's DSL List.
Canada - WHMIS
WHMIS: Not available.
This product has been classified in accordance with the hazard criteria of the Controlled Products
Regulations and the MSDS contains all of the information required by those regulations.
Canadian Ingredient Disclosure List
CAS# 10101-53-8 is listed on the Canadian Ingredient Disclosure List.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 The number of electrons in each of Chromium's shells is 2, 8, 13, 1 and its electron configuration is [Ar] 3d5 4s1. Louis Nicolas Vauquelin first discovered chromium in 1797 and first isolated it the following year. The chromium atom has a radius of 128 pm and a Van der Waals radius of 189 pm. In its elemental form, chromium has a lustrous steel-gray appearance.
The number of electrons in each of Chromium's shells is 2, 8, 13, 1 and its electron configuration is [Ar] 3d5 4s1. Louis Nicolas Vauquelin first discovered chromium in 1797 and first isolated it the following year. The chromium atom has a radius of 128 pm and a Van der Waals radius of 189 pm. In its elemental form, chromium has a lustrous steel-gray appearance.  Chromium is the hardest metallic element in the periodic table and the only element that exhibits antiferromagnetic ordering at room temperature, above which it transforms into a paramagnetic solid. The most common source of chromium is chromite ore (FeCr2O4). Due to its various colorful compounds, Chromium was named after the Greek word 'chroma.' meaning color.
Chromium is the hardest metallic element in the periodic table and the only element that exhibits antiferromagnetic ordering at room temperature, above which it transforms into a paramagnetic solid. The most common source of chromium is chromite ore (FeCr2O4). Due to its various colorful compounds, Chromium was named after the Greek word 'chroma.' meaning color.
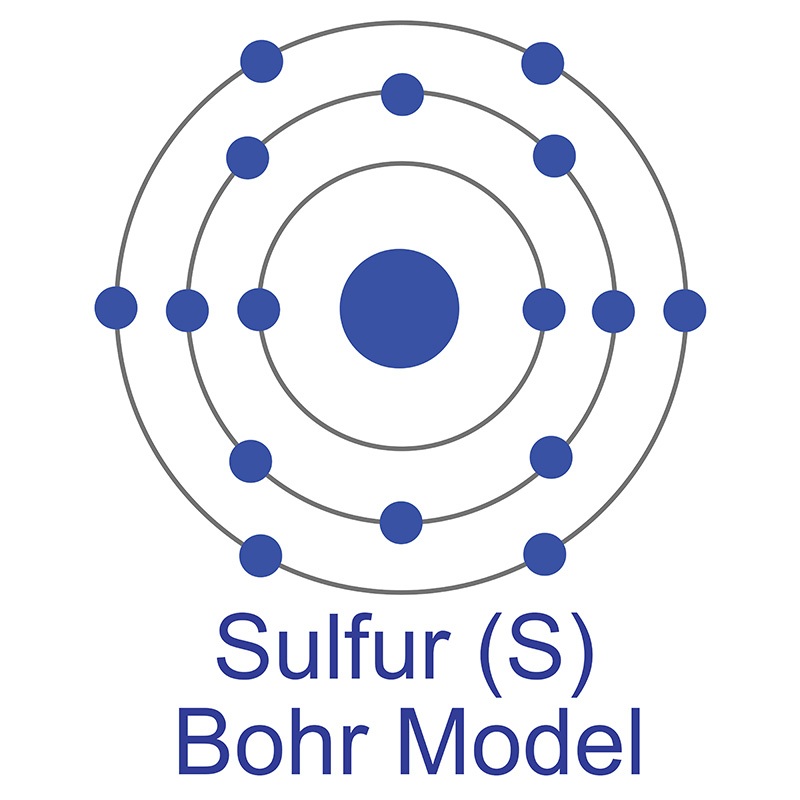 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.