SECTION 1. IDENTIFICATION
Product Name: Gadolinium Oxysulfide
Product Number: All applicable American Elements product codes, e.g. GD-OS-01-P
CAS #: 12339-07-0
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Signal Word: Warning
Pictograms:

Hazard Statements: H302 Harmful if swallowed.
H312 Harmful in contact with skin.
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H333 May be harmful if inhaled.
H335 May cause respiratory irritation.
Precautionary Statements: P260 Do not breath dust / fume / gas / mist / vapors / spray.
P264 Wash skin thoroughly after handling.
P270 Do not eat, drink or smoke when using this product.
P271 Use only outdoors or in a well-ventilated area.
P280 Wear protective gloves / protective clothing / eye protection / face protection.
P405 Store locked up.
P362+363 Take off contaminated clothing. Wash contaminated clothing before reuse.
P301+312 IF SHALLOWED: Call a POISON CENTER or physician if you feel unwell.
P305+351+338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing.
P302+332+313 IF ON SKIN: Wash with plenty of soap and water. If skin irritation occurs: get
medical advice/attention.
P337+313 If eye irritation persists get medical advice / attention.
P403+233 Store in a well ventilated place. Keep container tightly closed.
P304+340 IF INHALED: Remove person to fresh air and keep comfortable for breathing.
P501 Dispose of contents / container in accordance with local / regional / national / international
regulations.
HMIS Health Ratings (0-4)
- Health: 2
- Flammability: 1
- Physical: 1
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Additional Names: Gadolinium oxide sulfide; Digadolinium dioxide sulfide; Gadox
Percentage: 99+ wt%
CAS #: 12339-07-0
EC #: 235-598-4
SECTION 4. FIRST AID MEASURES
General Treatment Consult a physician. Show this SDS to the doctor in attendance. Move out of dangerous area.
Special Treatment: No Data Available.
Important Symptoms: Ingestion may cause nausea, vomiting, diarrhea, and abdominal pains. Inhalation of dust may
result in lung injury. Skin contact may cause a rash, redness or dermatitis. Eye contact may cause
redness or irritation.
Inhalation: If breathing, move person into fresh air. If not breathing, give artificial respiration. Consult a
physician. If irritation or other symptoms occur, remove from exposure and consult a physician
immediately.
Ingestion: Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a
physician.
Skin: For skin contact, flush with large amounts of soap and water while removing contaminated
clothing. Consult a physician.
Eyes: Immediately flush eyes with fresh water for at least 15 minutes while holding the eyelids open.
Remove contact lenses if worn. Consult a physician.
SECTION 5. FIREFIGHTING MEASURES
Flammability: Not Flammable.
Special Hazards from Substance: May evolve hazardous Sulfur Oxide fumes in fire.
Extinguishing Media: Use alcohol-resistant foam, dry chemical or carbon dioxide.
Special Fire Fighting Procedures: Wear self-contained breathing apparatus for firefighting if necessary
SECTION 6. ACCIDENTAL RELEASE MEASURES
If Material is Released / Spilled: Avoid dust formation. Avoid breathing vapors, mist, or gas. Ensure adequate ventilation. Vacuum
up spillage with vacuum equipped with appropriate filter to prevent airborne dust. If dust is present,
use NIOSH approved respirator. Collect in suitable container for disposal. Dispose of waste in
accordance with applicable federal, state, local, and provincial environmental regulations.
Environmental Precautions: Prevent further leakage or spills if safe to do so. Do not allow to enter drains, sewers, or
watercourses.
SECTION 7. HANDLING AND STORAGE
Handling Conditions: Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Provide appropriate
exhaust ventilation at places where dust is formed. For precautions see Section 2.
Storage Conditions: Keep container tightly closed in a cool, dry, and well-ventilated place.
Work / Hygienic Maintenance: Keep formation of airborne dusts to a minimum. Guard against dust accumulation. In case of
insufficient ventilation, wear suitable respiratory equipment.
Ventilation: Provide appropriate exhaust ventilation at places where dust is formed. In case of insufficient
ventilation wear suitable respiratory equipment.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Permissible Exposure Limits: Authority Basis Limit Remarks
- - - No Data Available.
Threshold Limit Value: Authority Basis Limit Remarks
- - - No Data Available.
Special Equipment: No Data Available.
Respiratory Protection: Where respirator if there is dust formation or high concentrations of material present. In case of
inadequate ventilation or risk of inhalation of dust, use suitable respiratory equipment.
Protective Gloves: Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching the glove’s outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
Eye Protection: Safety glasses and face shield. Use equipment for eye protection tested and approved under
government standards such as NIOSH (US) or EN 166 (EU).
Body Protection: Wear appropriate protective clothing. The use of bodily protective equipment must be determined
according to the concentration and amount of the dangerous substance at the specific workplace.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Color: White
Molecular Weight: 378.56 g/mol
Forms: Powder
Density: 7.32 g/cm3
Odor: Odorless
pH: No Data Available.
Water Solubility: Insoluble
Auto-Ignition Temperature: No Data Available.
Boiling Point: No Data Available.
Evaporation Rate: No Data Available.
Melting Point / Freezing Point: No Data Available.
Flammability or Explosive Limits: No Data Available.
Vapor Pressure: No Data Available.
Partition Coefficient: n-octanol/water: No Data Available.
Vapor Density: No Data Available.
Decomposition Temperature: No Data Available.
Flash Point: No Data Available.
Viscosity: No Data Available.
SECTION 10. STABILITY AND REACTIVITY
Stability: Stable under recommended storage conditions.
Reacts with: Material slowly hydrolyzes with formation of hydrogen sulfide in moist air and / or aqueous
solutions.
Incompatible Conditions: Strong acids, strong oxidizers, and flammable materials.
Hazardous Decomposition Products: Sulfur oxides, Hydrogen sulfide
SECTION 11. TOXICOLOGICAL INFORMATION
Potential Health Effects: Eyes: May cause irritation or redness.
Skin: May cause irritation, rash, redness, or dermatitis.
Ingestion: May cause irritation, nausea, vomiting, diarrhea, or abdominal pains.
Inhalation: May cause irritation, inhalation of dust may cause lung injury.
Chronic: No Data Available.
Signs and Symptoms: Lanthanons can cause delayed blood clotting leading to hemorrhages. Exposure may also lead to
sensitivity to heat, itching, increased awareness of odor and taste, and liver damage.
Aggravate Medical Conditions: Persons suffering from lung dysfunction should avoid exposure to dust.
Median Lethal Dose: No Data Available.
Carcinogen: IARC: No component of this product present at levels greater than or equal to 0.1% is
identified as probable or confirmed human carcinogen by IARC.
ACGIH: No component of this product present at levels greater than or equal to 0.1% is
identified as a carcinogen or potential carcinogen by ACGIH.
NTP: No component of this product present at levels greater than or equal to 0.1% is
identified as a known or anticipated carcinogen by NTP.
OSHA: No component of this product present at levels greater than or equal to 0.1% is
identified as a carcinogen or potential carcinogen by OSHA.
SECTION 12. ECOLOGICAL INFORMATION
Aquatic Toxicity: No Data Available.
Persistence and degradability: No Data Available.
Bioaccumulative potential: No Data Available.
Notes: Do not allow material to be released into the environment without proper government permits. Do
not allow undiluted product or large quantities to reach ground water, water course, or sewage
system. Avoid transfer into the environment.
SECTION 13. DISPOSAL CONSIDERATIONS
Disposal: Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Empty containers should be taken
to an appropriated waste handling site for recycling or disposal. Dispose of in accordance with
local, state, or national regulations.
SECTION 14. TRANSPORT INFORMATION
Hazardous:
DoT: Not Dangerous Goods
IMDG: Not Dangerous Goods
IATA: Not Dangerous Goods
Pictogram: N/A
Hazard Class: N/A
Packing Group: N/A
UN Number: N/A
Proper Shipping Name:
DoT: N/A
IMDG: N/A
IATA: N/A
SECTION 15. REGULATORY INFORMATION
SARA 302 Components
No chemical in this material are subject to the reporting requirements of SARA Title III,
Section 302.
SARA 313 Components
This material does not contain any chemical components with known CAS numbers that
exceed the threshold (de minimus) reporting levels established by SARA Title III, Section 313.
SARA 311/312 Hazards
Acute Health Hazard, Chronic Health Hazard
Massachusetts Right to Know Components
No components are subject to Ma. Right to Know Act.
Pennsylvania Right to Know Components
Gadolinium Oxysulfide (CAS No. 12339-07-0)
New Jersey Right to Know Components
Gadolinium Oxysulfide (CAS No. 12339-07-0)
California Prop. 65 Components
This product does not contain any chemicals known to the State of California to cause
cancer, birth defects, or any other reproductive harm.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
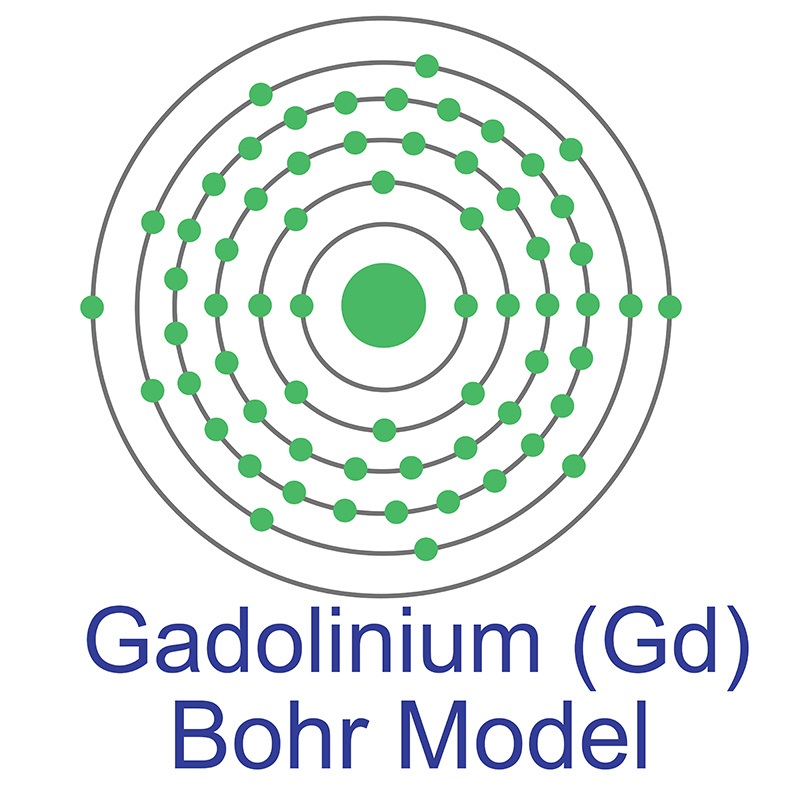 The number of electrons in each of Gadolinium's shells is [2, 8, 18, 25, 9, 2] and its electron configuration is [Xe] 4f7 5d1 6s2. The gadolinium atom has a radius of 180 pm and a Van der Waals radius of 237 pm. Gadolinium was discovered by Jean Charles Galissard de Marignac in 1880 and first isolated by Lecoq de Boisbaudran in 1886. In its elemental form, gadolinium has a silvery-white appearance. Gadolinium is a
The number of electrons in each of Gadolinium's shells is [2, 8, 18, 25, 9, 2] and its electron configuration is [Xe] 4f7 5d1 6s2. The gadolinium atom has a radius of 180 pm and a Van der Waals radius of 237 pm. Gadolinium was discovered by Jean Charles Galissard de Marignac in 1880 and first isolated by Lecoq de Boisbaudran in 1886. In its elemental form, gadolinium has a silvery-white appearance. Gadolinium is a  It is utilized for both its high magnetic moment (7.94μ B) and in
It is utilized for both its high magnetic moment (7.94μ B) and in 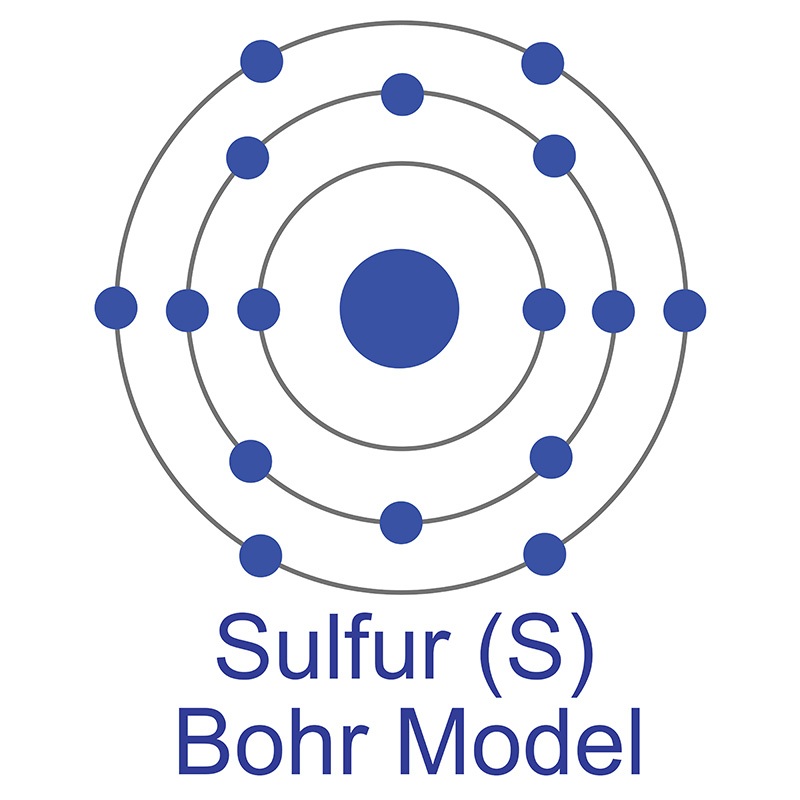 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.