About Gadolinium

Swiss chemist Jean Charles Galissard de Marignac discovered an oxide of an unknown element in a sample of gadolinite in 1880 and subsequently named the oxide “gadolinia”. The mineral had been named for Finnish chemist and geologist Johan Gadolin, who had discovered the first rare earth element, yttrium, in 1794. Thus gadolinium, named from its oxide, was only the second element to be named after an individual--the first being samarium, discovered just a year earlier, and which was also named after a mineral that had been named after a geologist.
Gadolinium has several properties that are key to its unique applications. It is probably best known for its magnetic properties. Gadolinium is ferromagnetic below 20 degrees Celsius and paramagnetic above this temperature. Due to these properties, gadolinium compounds are used to enhance contrast between normal and healthy tissue during magnetic resonance imaging (MRI) scanning. Gadolinium containing garnets have useful properties which lend them to applications in magneto-optical computer memory devices, however these types of computer memory have been replaced for most applications with alternative technologies that are faster and cheaper. Thin films of the magneto-optical material gadolinium-terbium-iron may also be use for these types of memory devices. Gadolinium also exhibits the magnetocaloric effect, meaning it experiences temperature changes when entering or exiting a magnetic field. This property is exploited gadolinium compounds used in magnetic refrigeration devices. Currently these devices are used primarily in research and industrial settings for ultra low temperature applications, however research developments may eventually make magnetic refrigeration a viable replacement for current commercial refrigeration technology.
An additional notable property of gadolinium is its high adsorption rate of neutrons. In nuclear energy applications, gadolinium is used in radiation shielding and in varying capacities to control the rate of the nuclear reaction. Gadolinium may also be used in neutron radiography, an imaging technology that uses neutrons much in the way x-rays are used in x-ray imaging. This technology is often used in industry for quality control when making precision parts. Gadolinium screens are used in neutron imaging to convert the neutrons that successfully pass through the imaged object into high-energy electrons which can then produce an image on x-ray film.
Gadolinium can also be used to produce compounds which exhibit luminescence either in response to the absorption of visible or near-visible light--these compounds are typically called phosphors--or in response to the absorption of ionizing radiation--these compounds are most often called scintillators. Gadolinium phosphors are used for green light in color display screens. Scintillator compounds containing gadolinium are used in sensors that detect X-rays or neutrons. These sensors are essential for the operation of medical imaging devices such as computed tomography scanners.
Gadolinium-153 is a radioactive isotope that is used in testing and calibration of medical imaging devices, bone density measurements, and in Lixiscope portable x-ray systems. Additionally, it has been investigated for potential use in radiotherapy for cancer.
In a few contexts, gadolinium is used in small quantities to alter the properties of a host material. In metallic alloys, gadolinium is used to to improve the workability of the material and increase resistance to high temperatures and oxidation. Gadolinium-doped garnets can be used in magneto-optical applications as previously mentioned, or may be used in microwave optical communications devices, lasers, or as imitation gemstones. Finally, gadolinium-doped ceria is an important potential electrolyte material for fuel cells, especially as it has higher ion conductivity and lower operating temperatures than the more commonly used yttria stabilized zirconia.
Several gadolinium compounds have been investigated for use as superconductors. Other uses currently in development for gadolinium include luminescent oxygen and temperature sensing compounds, novel high-k dielectrics for semiconductor devices, high temperature piezoelectric compounds for pressure and force detecting sensors, and compounds which can be used to immobilize and thus contain radioactive waste.
Gadolinium is a rare earth element that can be found in varying quantities in most rare-earth containing minerals. It is most commonly extracted from monazite and bastnasite.
Products
Gadolinium is utilized for both its high magnetic moment (7.94µB) and in phosphors and scintillator materials. When complexed with EDTA ligands, it is used as an  injectable contrast agent for patients undergoing magnetic resonance imaging. With its high magnetic moment, gadolinium can reduce
injectable contrast agent for patients undergoing magnetic resonance imaging. With its high magnetic moment, gadolinium can reduce  relaxation times and thereby enhance signal intensity. The extra stable half-full 4f electron shell with no low lying energy levels creates applications as an inert phosphor host. Gadolinium can therefore act as hosts for x-ray cassettes and in scintillator materials for computer tomography. Gadolinium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Gadolinium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
relaxation times and thereby enhance signal intensity. The extra stable half-full 4f electron shell with no low lying energy levels creates applications as an inert phosphor host. Gadolinium can therefore act as hosts for x-ray cassettes and in scintillator materials for computer tomography. Gadolinium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Gadolinium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Gadolinium Properties
 Gadolinium is a Block F, Group 3, Period 6 element.
Gadolinium is a Block F, Group 3, Period 6 element. 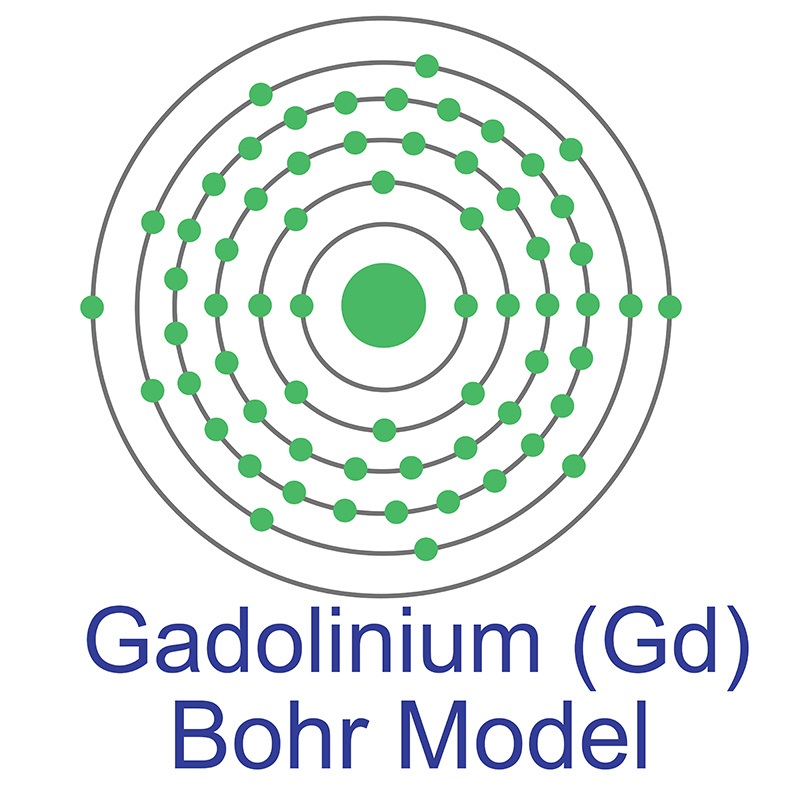 The number of electrons in each of Gadolinium's shells is 2, 8, 18, 25, 9, 2 and its electron configuration is [Xe] 4f7 5d1 6s2. The gadolinium atom has a radius of 180.pm and its Van der Waals radius is 237.pm. In its elemental form, CAS 7440-54-2, gadolinium has a silvery-white appearance. Gadolinium was discovered by Jean Charles Galissard de Marignac in 1880 and first isolated by Lecoq de Boisbaudran in 1886. The element is named after the Finnish chemist and geologist Johan Gadolin.
The number of electrons in each of Gadolinium's shells is 2, 8, 18, 25, 9, 2 and its electron configuration is [Xe] 4f7 5d1 6s2. The gadolinium atom has a radius of 180.pm and its Van der Waals radius is 237.pm. In its elemental form, CAS 7440-54-2, gadolinium has a silvery-white appearance. Gadolinium was discovered by Jean Charles Galissard de Marignac in 1880 and first isolated by Lecoq de Boisbaudran in 1886. The element is named after the Finnish chemist and geologist Johan Gadolin.
Health, Safety & Transportation Information for Gadolinium
Gadolinium can be very toxic. Safety data for Gadolinium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The following applies to elemental (metallic) Gadolinium.
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H261 |
| Hazard Codes | F |
| Risk Codes | 15 |
| Safety Precautions | 43 |
| RTECS Number | N/A |
| Transport Information | UN 3208 4.3 / PGIII |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Gadolinium Isotopes
Gadolinium (Gd) has 6 stable isotopes: 154Gd, 155Gd, 156Gd, 157Gd, 158Gd and 160Gd.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 134Gd | 133.95537(43)# | 0.4# s | Unknown | 0+ | N/A | 1057.32 | - |
| 135Gd | 134.95257(54)# | 1.1(2) s | Unknown | 3/2- | N/A | 1065.4 | - |
| 136Gd | 135.94734(43)# | 1# s [>200 ns] | ß+ to 136Eu | N/A | N/A | 1082.79 | - |
| 137Gd | 136.94502(43)# | 2.2(2) s | ß+ to 137Eu | 7/2+# | N/A | 1090.87 | - |
| 138Gd | 137.94012(21)# | 4.7(9) s | ß+ to 138Eu | 0+ | N/A | 1098.95 | - |
| 139Gd | 138.93824(21)# | 5.7(3) s | ß+ to 139Eu | 9/2-# | N/A | 1116.34 | - |
| 140Gd | 139.93367(3) | 15.8(4) s | ß+ to 140Eu | 0+ | N/A | 1124.42 | - |
| 141Gd | 140.932126(21) | 14(4) s | ß+ to 141Eu; ß+ + p to 140Sm | (1/2+) | N/A | 1132.5 | - |
| 142Gd | 141.92812(3) | 70.2(6) s | ß+ to 142Eu | 0+ | N/A | 1149.89 | - |
| 143Gd | 142.92675(22) | 39(2) s | ß+ to 143Eu | (1/2)+ | N/A | 1157.97 | - |
| 144Gd | 143.92296(3) | 4.47(6) min | ß+ to 144Eu | 0+ | N/A | 1166.05 | - |
| 145Gd | 144.921709(20) | 23.0(4) min | ß+ to 145Eu | 1/2+ | N/A | 1174.13 | - |
| 146Gd | 145.918311(5) | 48.27(10) d | EC to 146Eu | 0+ | N/A | 1191.53 | - |
| 147Gd | 146.919094(3) | 38.06(12) h | EC to 147Eu | 7/2- | 1 | 1199.6 | - |
| 148Gd | 147.918115(3) | 74.6(30) y | a to 144Sm | 0+ | N/A | 1207.68 | - |
| 149Gd | 148.919341(4) | 9.28(10) d | EC to 149Eu; a to 145Sm | 7/2- | 0.9 | 1215.76 | - |
| 150Gd | 149.918659(7) | 1.79(8)E+6 y | a to 146Sm | 0+ | N/A | 1223.84 | - |
| 151Gd | 150.920348(4) | 124(1) d | EC to 151Eu; a to 147Sm | 7/2- | 0.8 | 1222.6 | - |
| 152Gd | 151.9197910(27) | 1.08(8)E+14 y | a to 148Sm | 0+ | N/A | 1240 | 0.2 |
| 153Gd | 152.9217495(27) | 240.4(10) d | EC to 153Eu | 3/2- | 0.4 | 1238.76 | - |
| 154Gd | 153.9208656(27) | Observationally Stable | - | 0+ | N/A | 1246.84 | 2.18 |
| 155Gd | 154.9226220(27) | Observationally Stable | - | 3/2- | -0.2591 | 1254.92 | 14.8 |
| 156Gd | 155.9221227(27) | STABLE | - | 0+ | N/A | 1263 | 20.47 |
| 157Gd | 156.9239601(27) | STABLE | - | 3/2- | -0.3399 | 1271.08 | 15.65 |
| 158Gd | 157.9241039(27) | STABLE | - | 0+ | N/A | 1279.15 | 24.84 |
| 159Gd | 158.9263887(27) | 18.479(4) h | ß- to 159Tb | 3/2- | -0.44 | 1287.23 | - |
| 160Gd | 159.9270541(27) | Observationally Stable | - | 0+ | N/A | 1295.31 | 21.86 |
| 161Gd | 160.9296692(29) | 3.646(3) min | ß- to 161Tb | 5/2- | N/A | 1303.39 | - |
| 162Gd | 161.930985(5) | 8.4(2) min | ß- to 162Tb | 0+ | 2 | 1302.15 | - |
| 163Gd | 162.93399(32)# | 68(3) s | ß- to 163Tb | 7/2+# | N/A | 1310.23 | - |
| 164Gd | 163.93586(43)# | 45(3) s | ß- to 164Tb | 0+ | 1.9 | 1318.31 | - |
| 165Gd | 164.93938(54)# | 10.3(16) s | ß- to 165Tb | 1/2-# | N/A | 1326.39 | - |
| 166Gd | 165.94160(64)# | 4.8(10) s | ß- to 166Tb | 0+ | 1.8 | 1325.15 | - |
| 167Gd | 166.94557(64)# | 3# s | ß- to 167Tb | 5/2-# | N/A | 1333.23 | - |
| 168Gd | 167.94836(75)# | 300# ms | ß- to 168Tb | 0+ | 1.4 | 1341.31 | - |
| 169Gd | 168.95287(86)# | 1# s | ß- to 169Tb | 7/2-# | N/A | 1340.07 | - |