About Neodymium
In 1841, Carl Gustav Mosander obtained a novel oxide from his experiments with rare-earth containing minerals. He believed this compound to be an oxide of a new rare earth element that he called didymium. This name was derived from the greek didymos, meaning twin, because Mosander felt it had very similar properties to the rare earth element he had previously discovered, lanthanum. Didymium was included as an element on an early version of Dmitri Mendeleev’s periodic table. Later experiments by Austrian chemist Carl Auer Welsbach in 1885 showed that Mosander’s oxide was actually a mixture of salts of two new elements, which were subsequently named praseodymium and neodymium. “Dymium” was retained from the original name, and “neo” simply means “new”.
Neodymium magnets, made from an alloy of neodymium, iron, and boron, are the strongest known permanent magnets. They are essential for many modern electronics, including microphones, speakers, hard disks, and electric motors. The vast majority of neodymium's commercial use isin production of these high-strength magnets.
In most other applications of neodymium, it is added in small amounts to alter the properties of a host material. Neodymium oxide is used as a colorant to produce glass that varies in shade depending on what type of lighting it is viewed in, a property valued by collectors. The color change phenomenon results from the sharp absorption bands of light transmitted through neodymium glasses, a feature that makes the same glass useful for photography filters and in scientific settings. Additionally, neodymium is used in combination with praseodymium to produce glass for safety goggles that block the high intensity yellow light and ultraviolet and infrared light produced during welding or glass blowing. Neodymium is also an important component of a variety of gain media used in lasers operating at infrared wavelengths. Yttrium aluminum garnet, yttrium lithium fluoride, and yttrium orthovanadate crystals can all be neodymium doped for this purpose. Commercially available laser pointers generally use neodymium doped crystals to produce infrared light that is converted to green light. Additionally, neodymium glass can itself be a laser gain medium and is particularly useful in extremely high power lasers.
Neodymium is one of the light rare earths, and is typically sourced, along with other elements from that group, from the minerals monazite and bastnasite.
Products
 Primary applications include lasers, glass coloring and tinting, dielectrics and, most importantly, as the fundamental basis for neodymium-iron-boron permanent magnets. Neodymium has a strong absorption band centered at 580 nm, which is very close to the human eye's maximum level of sensitivity, making it useful in protective lenses for welding goggles. It is also used in CRT displays to enhance contrast between reds and greens and highly valued in glass manufacturing for its attractive purple coloring.
Primary applications include lasers, glass coloring and tinting, dielectrics and, most importantly, as the fundamental basis for neodymium-iron-boron permanent magnets. Neodymium has a strong absorption band centered at 580 nm, which is very close to the human eye's maximum level of sensitivity, making it useful in protective lenses for welding goggles. It is also used in CRT displays to enhance contrast between reds and greens and highly valued in glass manufacturing for its attractive purple coloring.  Neodymium is included in many formulations of barium titanate, used as dielectric coatings and in multi-layer capacitors essential to electronic equipment. Neodymium is available as metal and
Neodymium is included in many formulations of barium titanate, used as dielectric coatings and in multi-layer capacitors essential to electronic equipment. Neodymium is available as metal and  compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Neodymium oxide is available in powder and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Neodymium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Neodymium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Neodymium oxide is available in powder and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Neodymium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Neodymium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Neodymium Properties
 Neodymium is a Block F, Group 3, Period 6 element.
Neodymium is a Block F, Group 3, Period 6 element. 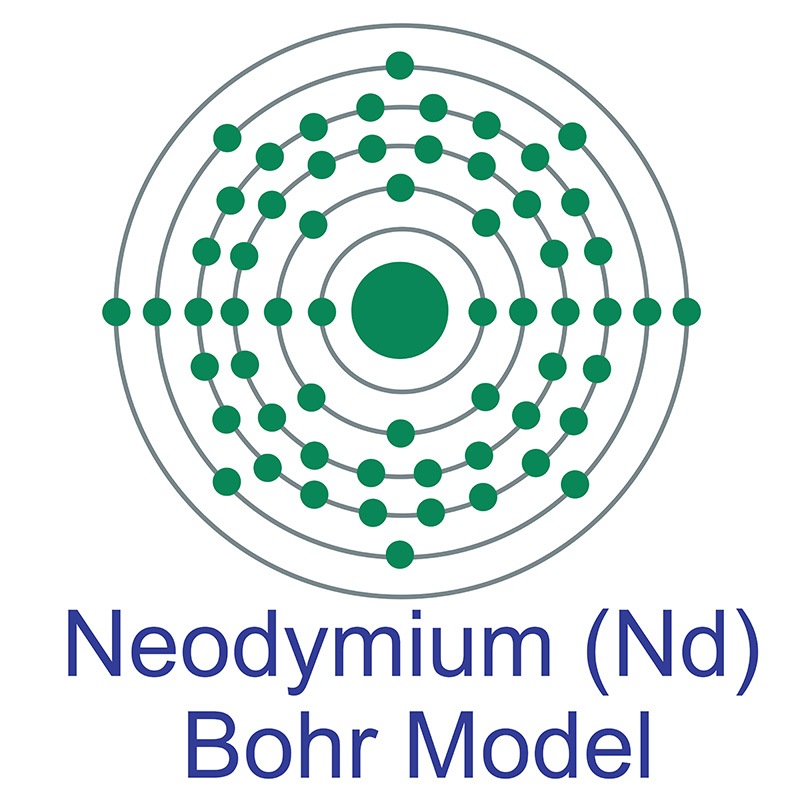 The number of electrons in each of Neodymium's shells is 2, 8, 18, 22, 8, 2 and its electron configuration is [Xe] 4f4 6s2. The neodymium atom has a radius of 181.pm and its Van der Waals radius is 229.pm. In its elemental form, CAS 7440-00-8, neodymium has a silvery-white appearance. Neodymium is the most abundant of the rare earths after cerium and lanthanum. Neodymium is found in monazite and bastnäsite ores. Neodymium was first discovered by Carl Aer von Welsbach in 1885. The name originates from the Greek words ‘neos didymos’, meaning new twin.
The number of electrons in each of Neodymium's shells is 2, 8, 18, 22, 8, 2 and its electron configuration is [Xe] 4f4 6s2. The neodymium atom has a radius of 181.pm and its Van der Waals radius is 229.pm. In its elemental form, CAS 7440-00-8, neodymium has a silvery-white appearance. Neodymium is the most abundant of the rare earths after cerium and lanthanum. Neodymium is found in monazite and bastnäsite ores. Neodymium was first discovered by Carl Aer von Welsbach in 1885. The name originates from the Greek words ‘neos didymos’, meaning new twin.
Health, Safety & Transportation Information for Neodymium
Safety data for Neodymium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Neodymium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H260-H315-H319-H335 |
| Hazard Codes | F,Xi |
| Risk Codes | 11-14/15-36/37/3 |
| Safety Precautions | 16-26-33-36/37/39-43 |
| RTECS Number | QO8575000 |
| Transport Information | UN 3208 4.3/PG |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Neodymium Isotopes
Neodymium has five stable isotopes: 142Nd, 143Nd, 145Nd, 146Nd and 148Nd.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 124Nd | 123.95223(64)# | 500# ms | Unknown | 0+ | N/A | 981.02 | - |
| 125Nd | 124.94888(43)# | 600(150) ms | Unknown | 5/2(+#) | N/A | 998.41 | - |
| 126Nd | 125.94322(43)# | 1# s [>200 ns] | ß+ to 126Pr | 0+ | N/A | 1006.49 | - |
| 127Nd | 126.94050(43)# | 1.8(4) s | ß+ to 127Pr | 5/2+# | N/A | 1014.57 | - |
| 128Nd | 127.93539(21)# | 5# s | ß+ to 128Pr | 0+ | N/A | 1031.97 | - |
| 129Nd | 128.93319(22)# | 4.9(2) s | ß+ to 129Pr | 5/2+# | N/A | 1040.04 | - |
| 130Nd | 129.92851(3) | 21(3) s | ß+ to 130Pr | 0+ | N/A | 1057.44 | - |
| 131Nd | 130.92725(3) | 33(3) s | ß+ to 131Pr | (5/2)(+#) | N/A | 1065.52 | - |
| 132Nd | 131.923321(26) | 1.56(10) min | ß+ to 132Pr | 0+ | N/A | 1073.6 | - |
| 133Nd | 132.92235(5) | 70(10) s | ß+ to 133Pr | (7/2+) | N/A | 1081.67 | - |
| 134Nd | 133.918790(13) | 8.5(15) min | ß+ to 134Pr | 0+ | N/A | 1099.07 | - |
| 135Nd | 134.918181(21) | 12.4(6) min | ß+ to 135Pr | 9/2(-) | N/A | 1107.15 | - |
| 136Nd | 135.914976(13) | 50.65(33) min | ß+ to 136Pr | 0+ | N/A | 1115.23 | - |
| 137Nd | 136.914567(12) | 38.5(15) min | ß+ to 137Pr | 1/2+ | N/A | 1123.31 | - |
| 138Nd | 137.911950(13) | 5.04(9) h | EC to 138Pr | 0+ | N/A | 1131.38 | - |
| 139Nd | 138.911978(28) | 29.7(5) min | EC to 139Pr | 3/2+ | N/A | 1139.46 | - |
| 140Nd | 139.90955(3) | 3.37(2) d | EC to 140Pr | 0+ | N/A | 1156.86 | - |
| 141Nd | 140.909610(4) | 2.49(3) h | EC to 141Pr | 3/2+ | 1.01 | 1164.94 | - |
| 142Nd | 141.9077233(25) | STABLE | - | 0+ | N/A | 1173.02 | 27.2 |
| 143Nd | 142.9098143(25) | Observationally Stable | - | 7/2- | -1.065 | 1181.09 | 12.2 |
| 144Nd | 143.9100873(25) | 2.29(16)E+15 y | a to 140Ce | 0+ | N/A | 1179.86 | 23.8 |
| 145Nd | 144.9125736(25) | Observationally Stable | - | 7/2- | -0.656 | 1187.94 | 8.3 |
| 146Nd | 145.9131169(25) | Observationally Stable | - | 0+ | N/A | 1196.01 | 17.2 |
| 147Nd | 146.9161004(25) | 10.98(1) d | ß- to 147Pm | 5/2- | 0.58 | 1204.09 | - |
| 148Nd | 147.916893(3) | Observationally Stable | - | 0+ | N/A | 1212.17 | 5.7 |
| 149Nd | 148.920149(3) | 1.728(1) h | ß- to 149Pm | 5/2- | 0.35 | 1210.93 | - |
| 150Nd | 149.920891(3) | 6.7(7)E+18 y | 2ß- to 150Sm | 0+ | N/A | 1219.01 | 5.6 |
| 151Nd | 150.923829(3) | 12.44(7) min | ß- to 151Pm | 3/2+ | N/A | 1227.09 | - |
| 152Nd | 151.924682(26) | 11.4(2) min | ß- to 152Pm | 0+ | N/A | 1235.17 | - |
| 153Nd | 152.927698(29) | 31.6(10) s | ß- to 153Pm | (3/2)- | N/A | 1243.25 | - |
| 154Nd | 153.92948(12) | 25.9(2) s | ß- to 154Pm | 0+ | N/A | 1251.33 | - |
| 155Nd | 154.93293(16)# | 8.9(2) s | ß- to 155Pm | 3/2-# | N/A | 1250.09 | - |
| 156Nd | 155.93502(22) | 5.49(7) s | ß- to 156Pm | 0+ | N/A | 1258.17 | - |
| 157Nd | 156.93903(21)# | 2# s [>300 ns] | ß- to 157Pm | 5/2-# | N/A | 1266.25 | - |
| 158Nd | 157.94160(43)# | 700# ms [>300 ns] | ß- to 158Pm | 0+ | N/A | 1265.01 | - |
| 159Nd | 158.94609(54)# | 500# ms | ß- to 159Pm | 7/2+# | N/A | 1273.09 | - |
| 160Nd | 159.94909(64)# | 300# ms | ß- to 160Pm | 0+ | N/A | 1281.17 | - |
| 161Nd | 160.95388(75)# | 200# ms | ß- to 161Pm | 1/2-# | N/A | 1279.93 | - |