About Carbon
Carbon is far from the most common element in the universe; at 0.5% it is less abundant than hydrogen, helium, and oxygen, yet it is arguably the most important element on earth. The basis for life as we define it, carbon is not only critical to all processes both biological and environmental, but is also the basis for all technology used by society, from energy for transportation to industrial manufacturing, and is playing an increasingly more important role in the advanced high technology semiconductors, electronics, nanotechnology, and green technology.
The diversity of uses for carbon is mirrored by its equally diverse number of forms. Elemental carbon, a nonmetal (though sometimes classified as a semimetal or metalloid), exists in several different structural forms known as allotropes that differ widely in appearance and properties: graphite and diamond, the most classically well-known forms, are only two of a set that has been expanded by additional recently discovered or artificially synthesized forms using advanced technology such as chemical vapor deposition.
Elemental Forms
Graphite is an opaque black form of carbon with a layered hexagonal crystal structure (in alpha form) that is extremely soft, thermodynamically stable, and electrically conductive. The beta form of graphite forms in the trigonal or rhombohedral lattice system; both forms have similar properties and can be converted via either mechanical or heat treatment. Graphite occurs naturally in various rocks and minerals around the world, but it can also be artificially synthesized by subjecting silicon carbide (or carborundum) to temperatures high enough to vaporize the silicon, leaving the graphitized carbon behind. It is perhaps most widely recognized as the soft gray “lead" in pencils. Its softness also makes it an excellent lubricant in powdered form. Because it is the most stable form of carbon under standard temperature and pressure ranges and remains intact even when subjected to extremely high temperatures, graphite often composes items such as heat shields, nuclear reactor components, and melting crucibles for high temperature processes such as metallurgy and crystal growth. Its other major metallurgic use is as an additive in steelmaking.
Pyrolytic graphite, or PG, is a chemically inert, high purity form of graphite artificially synthesized via chemical vapor deposition and considered to be a refractory ceramic material. It is extremely anisotropic, exhibiting both directional electrical and thermal conductivity, and extremely heat resistant, in addition to being lightweight and flexible; for these reasons it is typically commercially employed in coatings and heat spreaders for electronics components. Pyrolytic graphite also possesses the unusual property of diamagnetism, which causes it to float in mid-air above a high-powered magnet.
Black carbon, also known as soot or coal, was the earliest form recognized and utilized by humans. Coal has been used for thousands of years as fuel, and is still primarily applied as fuel today. This form of carbon usually has a black appearance, and varying structures depending on the amount of heat and pressure that has been applied to the material. There are a few different ranks of coal that depend on the pressurization such as Peat, Lignite, Sub-bituminous coal, Bituminous coal, Steam coal and Anthracite, with Anthracite as the highest rank. There are many different forms of this type of carbon including solids, powders and nanopowders.
Diamond is a transparent crystalline form of carbon and is the hardest known naturally occurring material on the planet. Though well known as a precious gemstone, like other gemstones such as sapphire (Al2O3), diamond has technical applications. Diamond saws are used to cut industrial materials, slice semiconductor wafers and single crystals. Diamonds are also used in the precision cutting and grinding of optical glass and steel. Though it is the hardest material known to man, the cost of the material and associated production costs are commercially prohibited. Diamond is often found in crystalline solid structures, but it can also be ground down into powders and nanopowders.
Amorphous carbon is a reactive form of carbon that lacks a defined crystal structure. Typically amorphous carbon refers to coal and other carbide derived carbons that are impure and neither graphite nor diamond. These forms contain significant amounts of other elements such as oxygen, hydrogen and sulfur. Amorphous carbon has a few applications such as antireflective coatings on crystalline silicon solar cells .
Glassy carbon, or vitreous carbon, is a non-graphitizing carbon which combines ceramic and glassy characteristics with those of graphite. This gives glassy carbon many important properties, such as high temperature resistance, hardness, low density, low electrical resistance, low friction, low thermal resistance, extreme resistance to chemical attack and impermeability to gases and liquids. One of the most prominent applications of glassy carbon is as an electrode material in electrochemistry. Other applications include use as high temperature crucibles and components in prosthetic devices.
Nanostructured Forms
Graphene is a two dimensional, single layer of carbon atoms in hexagonal lattice. It is the basis of other discovered two dimensional elemental forms such as hexagonal boron nitride, and the applications for these materials are numerous. Some of these include development in electronics, biological engineering, filtration, lightweight and strong composite materials, photovoltaics and energy storage. Graphene has many unique properties which include strength that is 200 times more than that of steel by weight, near transparence and extremely efficient heat and electrical conductivity. The material was first explored in 1947 by P.R. Wallace while he was studying three-dimensional graphite. However, extensive research on graphene was not prominent until the turn of the century. Graphene is also the basic structural element of other carbon allotropes including graphite, charcoal, carbon nanotubes and fullerenes. Other forms of graphene include graphene oxide, which has enhanced photo-conducting properties, and nitrogen-doped graphene, which features efficient growth, structure and electronic properties.
Carbon nanotubes are another allotrope of carbon with a cylindrical nanostructure. Nanotubes have a significantly larger length-to-diameter ratio than any other material, with ratios of up to 132,000,000:1 having been constructed. Carbon nanotubes have very unique properties that are valuable in fields such as nanotechnology, electronics, optics and other fields of technology and materials sciences. These properties include extraordinary thermal conductivity and other mechanical and electrical properties. Other properties appear depending on the radius and rolling angle of the nanotubes, either making it a metal or a semiconductor. Carbon nanotubes are classified according to their wall thickness, either single-walled or multi-walled. Carbon nanotubes were first discovered by L.V. Radushkevich and V.M. Lukyanovich in 1952.
Fullerenes, or buckeyballs, are molecules of carbon in the form of a hollow sphere, ellipsoid, tube and a variety of other shapes. Fullerenes have a similar structure to graphite, which is made up of stacked graphene sheets in linked hexagonal rings. The first fullerene molecule discovered was a buckminsterfullerene by Richard Smalley, Robert Curl, James Heath, Sean O’Brien and Harold Kroto in 1985. This discovery greatly expanded the amount of known carbon allotropes from just graphite, diamond and amorphous carbon to many different nanomaterials and other varieties of carbon. Fullerenes have many technological applications in materials science, electronics and nanotechnology.
There are more and more carbon nanomaterials that are being developed every day. These include nanohoops, nanobuds, nanohorns and many others that have a large variety of applications.
Organometallics & Metalorganics
Organometallic chemistry is the study of chemical compounds that have a minimum of one bond between a metal and a carbon atom of an organic compound. Organometallic chemistry incorporates characteristics of both inorganic and organic chemistry. Carbon, hydrogen and oxygen are the elements that make up organic molecules. When organic molecules are bonded to other atoms of the inorganic variety, molecules known as organometallics are created. The true classification of an organometallics is dependent on the specific location of the carbon bond. Molecules known as Organometallic refer to complexes in which the site of the carbon bond occurs with a metal atom. The first metal complex identified as an organometallic was Potassium trichloro(ethene)platinate(II) - or Zeise’s salt - which is a salt that was obtained from a reaction of ethylene with platinum(II) chloride. These materials have common properties such as low melting points, insolubility in water, solubility in ether, toxicity, oxidizability and high reactivity. When the metal is bonded to a different element like oxygen or hydrogen, the resulting material is classified roughly as a Metalorganic. Examples of organometallics include tetracarbonyl nickel, ferrocene, diethylmagnesium, iodo(methyl)magnesium and diethylzinc among others. Metalorganics include beta-diketonates, alkoxides, and dialkylamides.
The main applications of organometallics are in stoichiometric and catalytic processes. Organometallics are extremely useful as catalysts or reagents in the synthesis of pharmaceutical products and other organic compounds. The earliest application of Organometallics in biomedicine was with the discovery of organoarsenical Salvarsan, which was the first antisyphilitic agent. Metalorganics have applications in material science for metalorganic vapor deposition and sol-gel processing.
Compounds
Carbonates are salts of carbonic acid that consist of one carbon atom surrounded by three oxygen atoms, with two single bonds between the carbon atom and the two negative oxygen atoms, and one short double bond between the carbon atom and the remaining neutral oxygen atom. This allows the oxygen atoms to link with other metal atoms in order to form compounds such as calcium carbonate and magnesium carbonate. Carbonates can also refer to functional groups within larger molecules in organic chemistry. Some of the more important carbonates in organic chemistry are dimethyl carbonate, ethylene carbonate, propylene carbonate and triphosgene, and are referred to as organocarbonates. Organocarbonates are widely used as solvents such as those used in lithium batteries, which, due to their high polarity, can dissolve lithium salts.
Carbides are very strong and heat resistant materials used in industrial cutting, high melting crucibles and furnace equipment. Carbides are composed of carbon and a less electronegative element with examples such as calcium carbide, silicon carbide, tungsten carbide and iron carbide. Carbides are generally classified by chemical bonding type into four categories: Salt-like, covalent compounds, interstitial compounds and transition metal carbides. Additionally, carbides can have crystalline forms such as that of silicon carbide, which has applications in electronic equipment such as power electronic devices and light emitting diodes.
Other compound forms of carbon include cyanides, which are molecules that consist of a carbon atom triple bonded to a nitrogen atom, and carbonyls, which are formed with a carbon atom triple bonded to an oxygen atom. Cyanides have applications within industrial organic chemistry, the mining industry, medicine, pest control and as food additives. Carbonyls are used in metallurgy, as catalysts and as carbon monoxide releasing molecules.
Alloys
There are many carbon alloys, with the most common being steel. According to the American Iron and Steel Institute the true name for steel is in fact carbon steel. Steel has many applications but the most important are within the construction, transportation, energy, packaging and appliance industries. There is typically a small amount of carbon in steel, ranging from about .12% - 2.0%, but levels can be as low as .05% or as high as 3.0% depending on the intended application. As the carbon percentage rises, steel becomes harder and stronger, but sacrifices ductility.
Other alloys containing carbon include stellite, which is used in saw teeth, hardfacing and acid-resistant machine parts; cast iron, which is primarily used in kitchen appliances; pig iron, which is used to make ductile iron; spiegeleisen, which is used in steelmaking; staballoy, which are primarily used in munitions; and nickel-carbon.
Applications for Carbon
Carbon has many applications throughout an immense number of industries. The most prominent of these is the fossil fuel industry, where hydrocarbons in methane gas and petroleum are used for fuel. Other valuable sources of carbon are in carbon-containing polymers that are produced in plants in the form of cotton, linen and hemp. These carbon-containing polymers can also be procured from animals in the form of wool, cashmere and silk. Carbon-containing polymers have obvious applications in the clothing industry.
A new application for carbon that is being developed is within battery anodes. Carbon-coated particles are used specifically in lithium ion batteries, in which the carbon coating on the particles allow lithium ions to pass through freely, while limiting the movement of the particle within its shell. Carbon materials are also finding applications within the defense industry, with carbon nanotubes being substituted for copper wire and cables in aerospace and defense electronics due to its significantly lighter weight. Another carbon form that is finding new uses is carbon foam, which is applicable as a container for active materials with needs for thermal energy storage, electric energy storage, absorbents for large molecules and others including microwave absorption.
Products
Due to their extreme hardness and resistance to heat and pressure, diamond and diamond micropowder have numerous industrial applications in geological drilling  bits, grinding media and as a high-strength/ high-temperature abrasive. Carbon also finds application in steel alloys, in various filtering and purification technologies, and as a neutron moderator in nuclear power plants. Carbon is available in its elemental form and as compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Carbon nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Carbon is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
bits, grinding media and as a high-strength/ high-temperature abrasive. Carbon also finds application in steel alloys, in various filtering and purification technologies, and as a neutron moderator in nuclear power plants. Carbon is available in its elemental form and as compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Carbon nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Carbon is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Carbon Properties
 Carbon is a Block P, Group 14, Period 2 element.
Carbon is a Block P, Group 14, Period 2 element. 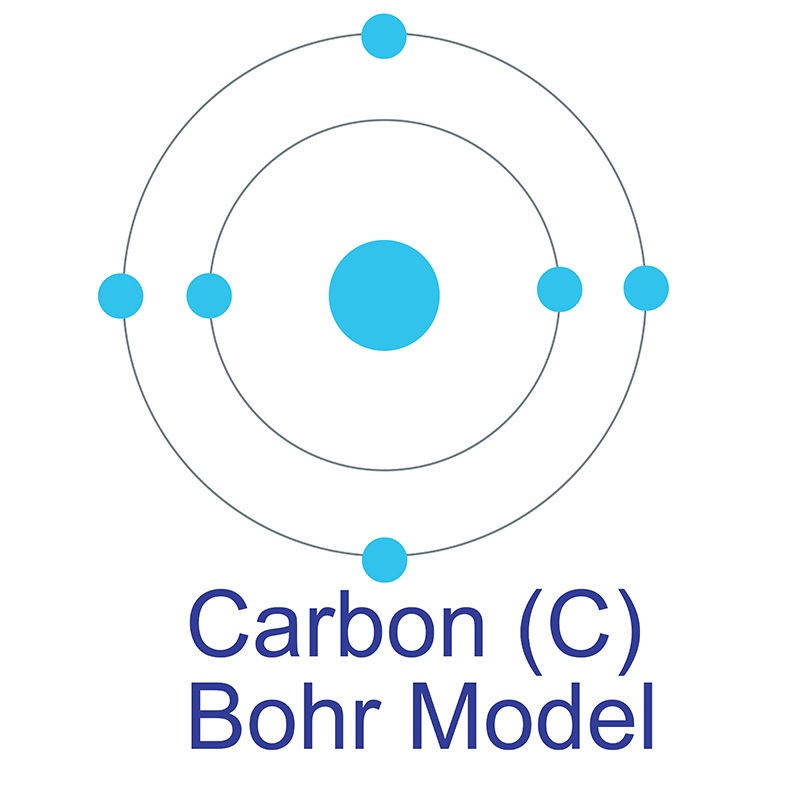 The number of electrons in each of Carbon's shells is 2, 4 and its electron configuration is [He] 2s2 2p2. In its elemental form, carbon's CAS number is 7440-44-0. Carbon is at the same time one of the softest (graphite) and hardest (diamond) materials found in nature. It is the 15th most abundant element in the Earth's crust, and the fourth most abundant element (by mass) in the universe after hydrogen, helium, and oxygen. Carbon was discovered by the Egyptians and Sumerians circa 3750 BC. It was first recognized as an element by Antoine Lavoisier in 1789.
The number of electrons in each of Carbon's shells is 2, 4 and its electron configuration is [He] 2s2 2p2. In its elemental form, carbon's CAS number is 7440-44-0. Carbon is at the same time one of the softest (graphite) and hardest (diamond) materials found in nature. It is the 15th most abundant element in the Earth's crust, and the fourth most abundant element (by mass) in the universe after hydrogen, helium, and oxygen. Carbon was discovered by the Egyptians and Sumerians circa 3750 BC. It was first recognized as an element by Antoine Lavoisier in 1789.
Health, Safety & Transportation Information for Carbon
Carbon in its purest form has very low toxicity. Carbon black dust, such as soot or coal dust, can cause irritation and damage to the lungs when inhaled in large quantities. Safety data for Carbon and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to graphitic carbon in bulk (non-powdered) form.
| Safety Data | |
|---|---|
| Signal Word | N/A |
| Hazard Statements | N/A |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | FF5250100 |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
N/A |
Carbon Isotopes
Carbon has two stable isotopes: 12C and 13C.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 8C | 8.037675(25) | 2.0(4) x 10-21 s [230(50) keV] | 2p to 6Be | 0+ | N/A | 22.87 | - |
| 9C | 9.0310367(23) | 126.5(9) ms | EC to 9B; EC + p to 8Be; EC + 2a to 2H | (3/2-) | N/A | 37.1 | - |
| 10C | 10.0168532(4) | 19.290(12) s | EC to 10B | 0+ | N/A | 59.15 | - |
| 11C | 11.0114336(10) | 20.334(24) min | EC to 11B | 3/2- | -0.964 | 71.89 | - |
| 12C | 12 exactly | STABLE | - | 0+ | 0 | 90.21 | 98.93 |
| 13C | 13.0033548378(10) | STABLE | - | 1/2- | 0.702411 | 95.5 | 1.07 |
| 14C | 14.003241989(4) | 5.70(3) x 103 yeyrs | ß- to 14N | 0+ | N/A | 103.57 | - |
| 15C | 15.0105993(9) | 2.449(5) s | ß- to 15N | 1/2+ | 1.32 | 105.13 | - |
| 16C | 16.014701(4) | 0.747(8) s | ß- to 16N | 0+ | N/A | 109.48 | - |
| 17C | 17.022586(19) | 193(5) ms | ß- to 17N; ß- + n to 16N | (3/2+) | N/A | 110.11 | - |
| 18C | 18.02676(3) | 92(2) ms | ß- to 18N; ß- + n to 17N | 0+ | N/A | 114.46 | - |
| 19C | 19.03481(11) | 46.2(23) ms | ß- + n to 18N; ß- to 19N; ß- + 2n to 17N | (1/2+) | N/A | 115.09 | - |
| 20C | 20.04032(26) | 16(3) ms [14(+6-5) ms] | ß- + n to 19N; ß- to 20N | 0+ | N/A | 117.58 | - |
| 21C | 21.04934(54)# | <30 ns | n to 20C | (1/2+)# | N/A | 117.27 | - |
| 22C | 22.05720(97)# | 6.2(13) ms [6.1(+14-12) ms] | ß- to 22N | 0+ | N/A | 117.9 | - |