About Potassium
At 2.4% by mass of the earth’s crust, potassium is the 7th most abundant element in the earth’s crust, present in seawater, most soils, and minerals such as orthoclase, sylvite, carnallite, kainite, and langbeinite. Elemental potassium is typical of the alkali metals: it is a waxy solid soft enough to be cut with a knife, initially silvery in appearance, but rapidly darkens when exposed to oxygen in the air. It is extremely reactive because of its high electropositivity and will explode violently in contact with water, quickly converting to potassium hydroxide and liberating hydrogen gas. Potassium is the second least dense element that is solid at room temperature (behind lithium), and it is one of only three elements (with sodium and lithium) capable of floating on hydrocarbon-based mineral oil. Though potassium is nontoxic, it is classified as a hazardous material because of its dangerous reaction with water. Additionally, naturally occurring potassium is composed of three isotopes, two stable and one radioactive, and as such it is the most common radioactive element present in the human body.
For centuries prior to the element’s discovery, humans had used potash (potassium carbonate) in glass, soaps, and other applications, obtaining the compound by leaching wood ashes. In 1807, Sir Humphry Davy isolated metallic potassium from molten potassium hydroxide (caustic potash) using electrolysis. The element derives its name from potash and its elemental symbol “K” from the neo-Latin kalium and the Arabic al-qalyah or qali, meaning “alkali.” Currently, pure potassium metal is often produced in a similar electrolytic reaction of sodium metal and potassium chloride. Potassium compounds such as potassium chloride (often referred to as potash, muriate of potash, or MOP in the mineral industry), hydroxide, nitrate, carbonate, chloride, bromide, iodide, and sulfate are abundant and easily extracted from deposits in ancient lakes and seabeds, making them extremely commercially viable for industrial use. These compounds are highly soluble and are components of many soaps and detergents, glass and ceramic glazes, stains, and dyes. Compounds like potassium nitrate are used in gunpowder and pyrotechnics to produce the violet color in fireworks. High purity potassium compounds have numerous pharmacological, medical, and electronics applications. Sodium and potassium alloys are useful as heat transfer mediums, desiccants, reducing agents, and can be used in nuclear reactors.
Potassium is essential to life: the uptake of potassium ions is one of the key mechanisms of the human body and as such, compounds are important dietary components and can be used in food additives such as baking powder (potassium sodium tartrate) and low-sodium salt substitutes. Plants utilize soil-based potassium as a nutrient, and one of the main commercial uses of potassium compounds such as chloride, sulfate, and nitrate is in the production of fertilizers.
Additionally, experiments with potassium have shed light on several potentially useful applications for the element in the field of high technology. Potassium-ion batteries are an experimental design based on lithium-ion technology that substitute ions of potassium for those of lithium and have shown to retain charge after nearly 40 times more cycles than a similar lithium-ion model. Researchers have constructed a negative temperature system by cooling potassium gas to one billionth of a degree below absolute zero, a highly unusual achievement that could allow for further study of quantum effects in practical applications such as superconductivity.
Products
 High purity potassium compounds have numerous pharmacological, medical, and electronics applications.
High purity potassium compounds have numerous pharmacological, medical, and electronics applications.  Important compounds include the chloride (often referred to as potash, muriate of potash, or MOP in the mineral industry), hydroxide, nitrate, carbonate, chloride, bromide, iodide, and sulfate. Lower purity compounds are used in pyrotechnics for its violet color on ignition and in glass and ceramic glazes to produce this color. Potassium is also used as a nutrient in plant growth. Potassium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).Elemental or metallic forms include pellets, wire and granules for evaporation source material purposes. Potassium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Potassium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Potassium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Important compounds include the chloride (often referred to as potash, muriate of potash, or MOP in the mineral industry), hydroxide, nitrate, carbonate, chloride, bromide, iodide, and sulfate. Lower purity compounds are used in pyrotechnics for its violet color on ignition and in glass and ceramic glazes to produce this color. Potassium is also used as a nutrient in plant growth. Potassium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).Elemental or metallic forms include pellets, wire and granules for evaporation source material purposes. Potassium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Potassium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Potassium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Potassium Properties
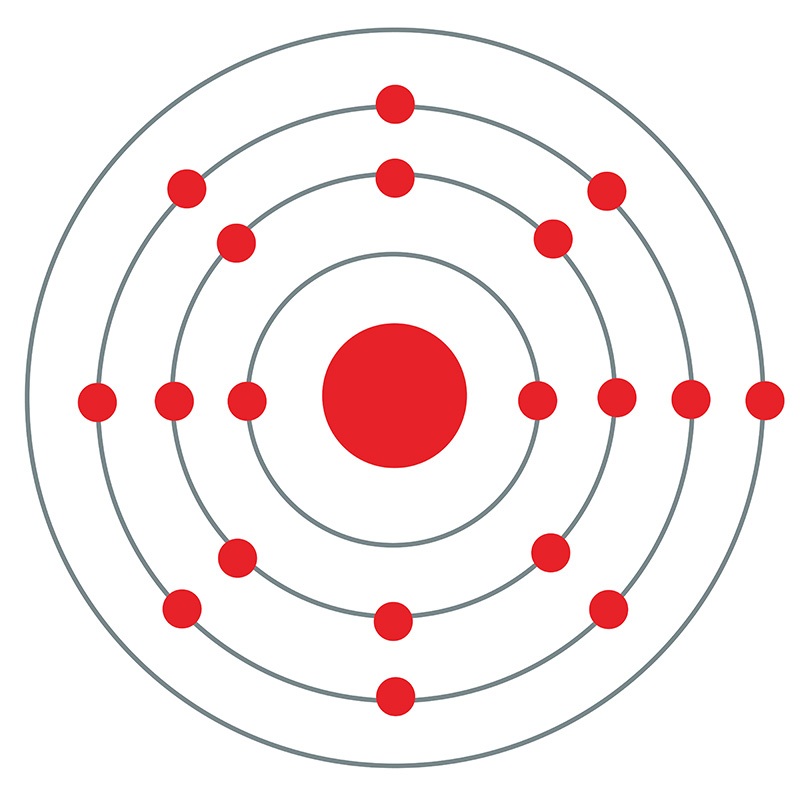
 Potassium is a Block S, Group 1, Period 4 element. The number of electrons in each of Potassium's shells is 2, 8, 8, 1 and its electron configuration is [Ar] 4s1.The potassium atom has a radius of 227.2 pm and its Van der Waals radius is 275 pm. In its elemental form, CAS 7440-09-7, potassium has a silvery gray appearance. Potassium is the seventh most abundant element on earth.
Potassium is a Block S, Group 1, Period 4 element. The number of electrons in each of Potassium's shells is 2, 8, 8, 1 and its electron configuration is [Ar] 4s1.The potassium atom has a radius of 227.2 pm and its Van der Waals radius is 275 pm. In its elemental form, CAS 7440-09-7, potassium has a silvery gray appearance. Potassium is the seventh most abundant element on earth.  It is one of the most reactive and electropositive of all metals and rapidly oxidizes.
It is one of the most reactive and electropositive of all metals and rapidly oxidizes. 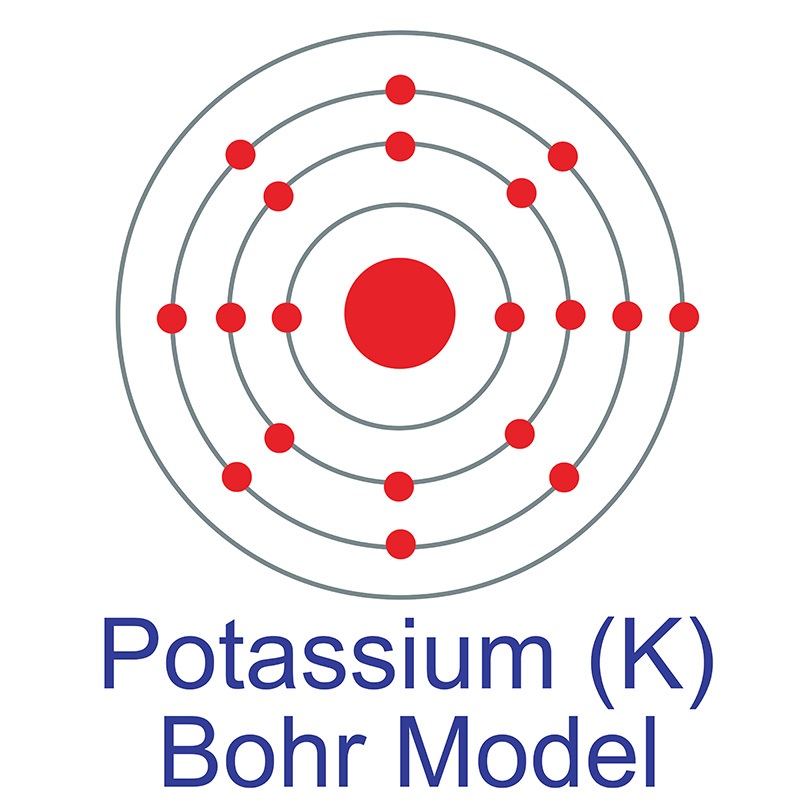 high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
Health, Safety & Transportation Information for Potassium
Potassium is not toxic in its elemental form; however, safety data for its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Potassium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H260-H314 |
| Hazard Codes | F,C |
| Risk Codes | 14/15-34 |
| Safety Precautions | 8-43-45 |
| RTECS Number | N/A |
| Transport Information | UN 2257 4.3/PG 1 |
| WGK Germany | 1 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Potassium Isotopes
Potassium (K) has 25 isotopes ranging from 32K to 56K. Three of these occur naturally: 39K (93.3%), 41K (6.7%), and 40K (0.012%).
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 32K | 32.02192(54)# | Unknown | p to 31Ar | 1+# | N/A | 217.64 | - |
| 33K | 33.00726(21)# | <25 ns | p to 32Ar | (3/2+)# | N/A | 238.76 | - |
| 34K | 33.99841(32)# | <40 ns | p to 33Ar | 1+# | N/A | 255.22 | - |
| 35K | 34.988010(21) | 178(8) ms | ß- to 35Ar; ß- + p to 33Cl | 3/2+ | N/A | 272.62 | - |
| 36K | 35.981292(8) | 342(2) ms | ß- to 36Ar; ß- + p to 35Cl; ß- + a to 32S | 2+ | N/A | 287.22 | - |
| 37K | 36.97337589(10) | 1.226(7) s | EC to 37Ar | 3/2+ | 0.2032 | 302.75 | - |
| 38K | 37.9690812(5) | 7.636(18) min | EC to 38Ar | 3+ | 1.37 | 314.55 | - |
| 39K | 38.96370668(20) | STABLE | - | 3/2+ | 0.3914658 | 328.22 | 93.2581 |
| 40K | 39.96399848(21) | 1.248(3)E+9 y | EC to 40Ar | 4- | -1.298099 | 336.3 | 0.0117 |
| 41K | 40.96182576(21) | STABLE | - | 3/2+ | 0.2148699 | 346.24 | 6.7302 |
| 42K | 41.96240281(24) | 12.360(12) h | ß- to 42Ca | 2- | -1.1425 | 353.39 | - |
| 43K | 42.960716(10) | 22.3(1) h | ß- to 43Ca | 3/2+ | 0.163 | 363.33 | - |
| 44K | 43.96156(4) | 22.13(19) min | ß- to 44Ca | 2- | -0.856 | 370.48 | - |
| 45K | 44.960699(11) | 17.3(6) min | ß- to 45Ca | 3/2+ | 0.1734 | 379.49 | - |
| 46K | 45.961977(17) | 105(10) s | ß- to 46Ca | 2(-) | -1.05 | 386.63 | - |
| 47K | 46.961678(9) | 17.50(24) s | ß- to 47Ca | 1/2+ | 1.93 | 394.71 | - |
| 48K | 47.965514(26) | 6.8(2) s | ß- to 48Ca;ß- + n to 47Ca | (2-) | N/A | 399.07 | - |
| 49K | 48.96745(8) | 1.26(5) s | ß- to 49Ca;ß- + n to 48Ca | (3/2+) | N/A | 405.28 | - |
| 50K | 49.97278(30) | 472(4) ms | ß- to 50Ca;ß- + n to 49Ca | (0-,1,2-) | N/A | 408.7 | - |
| 51K | 50.97638(54)# | 365(5) ms | ß- to 51Ca;ß- + n to 50Ca | 3/2+# | N/A | 413.05 | - |
| 52K | 51.98261(75)# | 105(5) ms | ß- + n to 51Ca; ß- + 2n to 50Ca; ß- to 52Ca | (2-)# | N/A | 415.54 | - |
| 53K | 52.98712(75)# | 30(5) ms | ß- + n to 52Ca; ß- + 2n to 51Ca; ß- to 53Ca | (3/2+)# | N/A | 418.96 | - |
| 54K | 53.99420(97)# | 10(5) ms | ß- to 54Ca;ß- + n to 53Ca | 2-# | N/A | 420.52 | - |
| 55K | 54.99971(107)# | 3# ms | ß- to 55Ca;ß- + n to 54Ca | 3/2+# | N/A | 423.94 | - |
| 56K | 56 | Unknown | Unknown | N/A | N/A | N/A | - |