SECTION 1. IDENTIFICATION
Product Name: Ultra Dry Hafnium(IV) Iodide
Product Number: All applicable American Elements product codes, e.g. HF4-I-02-P.UD
, HF4-I-03-P.UD
, HF4-I-04-P.UD
, HF4-I-05-P.UD
CAS #: 13777-23-6
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification
This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200)
Label Elements
Signal Word
Danger
Hazard Statements
Causes severe skin burns and eye damage
May cause respiratory irritation


Precautionary Statements
Prevention
Do not breathe dust/fume/gas/mist/vapors/spray
Wash face, hands and any exposed skin thoroughly after handling
Wear protective gloves/protective clothing/eye protection/face protection
Use only outdoors or in a well-ventilated area
Response
Immediately call a POISON CENTER or doctor/physician
Inhalation
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
Skin
IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower
Wash contaminated clothing before reuse
Eyes
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
Ingestion
IF SWALLOWED: Rinse mouth. DO NOT induce vomiting
Storage
Store locked up
Store in a well-ventilated place. Keep container tightly closed
Disposal
Dispose of contents/container to an approved waste disposal plant
Hazards not otherwise classified (HNOC)
None identified
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component - Hafnium iodide (HfI4)
CAS-No - 13777-23-6
Weight % - <=100
SECTION 4. FIRST AID MEASURES
General Advice Show this safety data sheet to the doctor in attendance. Immediate medical attention is
required.
Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes.
Immediate medical attention is required. Keep eye wide open while rinsing.
Skin Contact Wash off immediately with soap and plenty of water while removing all contaminated
clothes and shoes. Call a physician immediately.
Inhalation Remove to fresh air. If not breathing, give artificial respiration. Call a physician or poison
control center immediately. Do not use mouth-to-mouth method if victim ingested or inhaled
the substance; give artificial respiration with the aid of a pocket mask equipped with a
one-way valve or other proper respiratory medical device.
Ingestion Immediate medical attention is required. Do NOT induce vomiting. Drink plenty of water.
Never give anything by mouth to an unconscious person.
Most important symptoms and
effects
Causes burns by all exposure routes. Product is a corrosive material. Use of gastric
lavage or emesis is contraindicated. Possible perforation of stomach or esophagus should
be investigated: Ingestion causes severe swelling, severe damage to the delicate tissue
and danger of perforation
Notes to Physician Treat symptomatically
SECTION 5. FIREFIGHTING MEASURES
Suitable Extinguishing Media CO 2, dry chemical, dry sand, alcohol-resistant foam.
Unsuitable Extinguishing Media No information available
Flash Point No information available
Method - No information available
Autoignition Temperature No information available
Explosion Limits
Upper No data available
Lower No data available
Sensitivity to Mechanical Impact No information available
Sensitivity to Static Discharge No information available
Specific Hazards Arising from the Chemical
The product causes burns of eyes, skin and mucous membranes.
Hazardous Combustion Products
None known.
Protective Equipment and Precautions for Firefighters
As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full
protective gear. Thermal decomposition can lead to release of irritating gases and vapors.
NFPA
Health - 3
Flammability - 0
Instability - 0
Physical hazrds -
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal Precautions Use personal protective equipment as required. Evacuate personnel to safe areas. Avoid
contact with skin, eyes or clothing.
Environmental Precautions Should not be released into the environment. Do not allow material to contaminate ground
water system. See Section 12 for additional Ecological Information.
Methods for Containment and Clean
Up
Sweep up and shovel into suitable containers for disposal. Avoid dust formation.
SECTION 7. HANDLING AND STORAGE
Handling Wear personal protective equipment/face protection. Do not get in eyes, on skin, or on
clothing. Use only under a chemical fume hood. Do not breathe dust. Do not ingest. If
swallowed then seek immediate medical assistance.
Storage Corrosives area. Keep containers tightly closed in a dry, cool and well-ventilated place.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Engineering Measures Ensure that eyewash stations and safety showers are close to the workstation location.
Personal Protective Equipment
Eye/face Protection Wear appropriate protective eyeglasses or chemical safety goggles as described by
OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard
EN166.
Skin and body protection Wear appropriate protective gloves and clothing to prevent skin exposure.
Respiratory Protection Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard
EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if
exposure limits are exceeded or if irritation or other symptoms are experienced.
Hygiene Measures Handle in accordance with good industrial hygiene and safety practice.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical State Solid
Appearance Yellow
Odor No information available
Odor Threshold No information available
pH No information available
Melting Point/Range 400 °C / 752 °F
Boiling Point/Range No information available
Flash Point No information available
Evaporation Rate Not applicable
Flammability (solid,gas) No information available
Flammability or explosive limits
Upper No data available
Lower No data available
Vapor Pressure No information available
Vapor Density Not applicable
Specific Gravity No information available
Solubility No information available
Partition coefficient; n-octanol/water No data available
Autoignition Temperature No information available
Decomposition Temperature No information available
Viscosity Not applicable
SECTION 10. STABILITY AND REACTIVITY
Reactive Hazard None known, based on information available
Stability Stable under normal conditions.
Conditions to Avoid Incompatible products.
Incompatible Materials Strong oxidizing agents
Hazardous Decomposition ProductsNone under normal use conditions
Hazardous Polymerization Hazardous polymerization does not occur.
Hazardous Reactions None under normal processing.
SECTION 11. TOXICOLOGICAL INFORMATION
Acute Toxicity
Product Information
Component Information
Toxicologically Synergistic
Products
No information available
Delayed and immediate effects as well as chronic effects from short and long-term exposure
Irritation No information available
Sensitization No information available
Carcinogenicity The table below indicates whether each agency has listed any ingredient as a carcinogen.
Mutagenic Effects No information available
Reproductive Effects No information available.
Developmental Effects No information available.
Teratogenicity No information available.
STOT - single exposure Respiratory system
STOT - repeated exposure None known
Aspiration hazard No information available
Symptoms / effects,both acute and
delayed
Product is a corrosive material. Use of gastric lavage or emesis is contraindicated.
Possible perforation of stomach or esophagus should be investigated: Ingestion causes
severe swelling, severe damage to the delicate tissue and danger of perforation
Endocrine Disruptor Information No information available
Other Adverse Effects The toxicological properties have not been fully investigated
SECTION 12. ECOLOGICAL INFORMATION
Ecotoxicity
Do not empty into drains.
Persistence and Degradability No information available
Bioaccumulation/ Accumulation No information available.
Mobility No information available.
SECTION 13. DISPOSAL CONSIDERATIONS
Waste Disposal Methods Chemical waste generators must determine whether a discarded chemical is classified as a
hazardous waste. Chemical waste generators must also consult local, regional, and
national hazardous waste regulations to ensure complete and accurate classification.
SECTION 14. TRANSPORT INFORMATION
DOT
UN-No UN3260Proper Shipping Name Corrosive solid, acidic, inorganic, n.o.s.
Technical Name (Hafnium iodide)
Hazard Class 8
Packing Group II
TDG
UN-No UN3260
Proper Shipping Name Corrosive solid, acidic, inorganic, n.o.s.
Hazard Class 8
Packing Group II
IATA
UN-No UN3260
Proper Shipping Name Corrosive solid, acidic, inorganic, n.o.s.
Hazard Class 8
Packing Group II
IMDG/IMO
UN-No UN3260
Proper Shipping Name Corrosive solid, acidic, inorganic, n.o.s.
Hazard Class 8
Packing Group II
SECTION 15. REGULATORY INFORMATION
TSCA 12(b) - Notices of Export Not applicable
U.S. Federal Regulations
SARA 313 Not applicable
SARA 311/312 Hazard Categories See section 2 for more information
CWA (Clean Water Act) Not applicable
Clean Air Act Not applicable
OSHA - Occupational Safety and
Health Administration
Not applicable
CERCLA Not applicable
California Proposition 65 This product does not contain any Proposition 65 chemicals.
U.S. State Right-to-Know
Regulations
Not applicable
U.S. Department of Transportation
Reportable Quantity (RQ): N
DOT Marine Pollutant N
DOT Severe Marine Pollutant N
U.S. Department of Homeland
Security
This product does not contain any DHS chemicals.
Other International Regulations
Mexico - Grade No information available
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
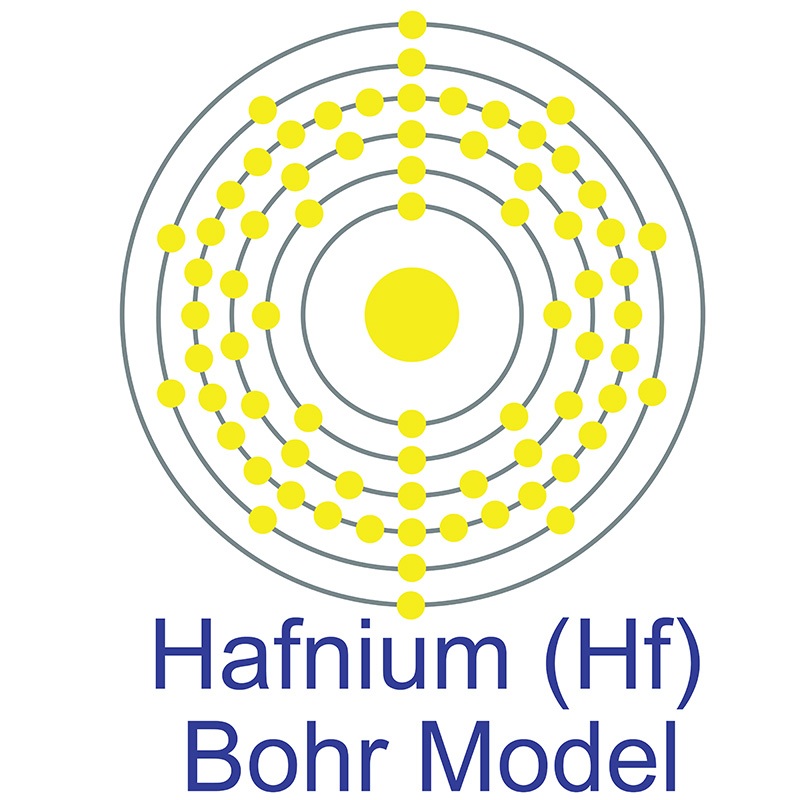 The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electron configuration is [Xe] 4f14 5d2 6s2. The hafnium atom has a radius of 159 pm and a Van der Waals radius of 212 pm. Hafnium was predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy. In its elemental form, hafnium has a lustrous silvery-gray appearance.
The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electron configuration is [Xe] 4f14 5d2 6s2. The hafnium atom has a radius of 159 pm and a Van der Waals radius of 212 pm. Hafnium was predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy. In its elemental form, hafnium has a lustrous silvery-gray appearance.  Hafnium does not exist as a free element in nature. It is found in
Hafnium does not exist as a free element in nature. It is found in