About Strontium
In 1790 a Scottish physician named Adair Crawford was the first to distinguish strontium-containing minerals from barium minerals. He had examined a supposed barium carbonate sample from the mine at Strontian, Argyllshire and upon finding that the sample lacked the expected chemical properties, proposed that he had found a new “earth”--a novel natural compound that likely contained a previously unknown element. Many of his contemporaries confirmed the results of his experiments and supported his assessment, and the name “strontium” was given to the new element based upon the origins of the source mineral. It was not until 1808 that Sir Humphry Davy succeeded in isolating the pure metal, a difficult task due to strontium’s high reactivity with water and air. He ultimately succeeded using electrolysis of a mixture of mercury and strontium oxides, a method that also allowed him to produce barium and calcium metals for the first time.
Strontium’s earliest industrial use was in the extraction of sugar from sugar beet molasses through a chemical procedure known as the Strontian process. This method is now obsolete, but strontium remains in use primarily due to the unique chemistry of its compounds. Strontium salts typically burn a bright red, and for this strontium compounds, especially strontium nitrate, are included in fireworks, flares, and tracer ammunition. Strontium oxide is used in pottery glazes to replace alternative compounds which contain toxic lead or barium. When added to glass, the same compound increases hardness, strength, and the index of refraction. This produces a higher-quality glass suitable for optical applications. Additionally, strontium glass blocks UV and X-ray radiation, and for this reason is included in cathode ray tube (CRT) display faceplates. This was once one of the largest uses of strontium, before the use of CRT displays began to decline. Strontium ferrite is a ceramic compound used to produce high strength magnets. These magnets are useful for their resistance to corrosion, low density, effectiveness at high-temperatures, and their ability to be very permanently magnetized. They are generally found in small motors, speakers, decorative magnets, and toys.
Strontium additionally has important medical uses resulting from its chemical similarities to calcium. Though not needed in the body, strontium is taken up by the bones just like calcium, and is known to promote calcium uptake, increasing bone density. Strontium ranelate is used as a drug treatment for patients with osteoporosis, and succeeds in strengthening bones and preventing breaks. Additionally, the radioisotope strontium-89 is used to treat pain from metastatic bone cancer, as it will localize to the bones naturally and kill the cancer cells there, stopping the extreme pain caused by tumors growing within the bones. Strontium gels have even been investigated as promoters of bone growth in bone tissue engineering. Strontium chloride also serves a medical function, as it treats tooth sensitivity when included in toothpaste.
Strontium metal is used fairly rarely, but it is added to some aluminum alloys to improve their ability to be cast into detailed structures, and it also finds occasional use as a chemical reagent.
Strontium, like its fellow alkali earths, is highly reactive, and therefore is never found naturally in the form of the pure metal. Its primary mineral deposits are celestite, a strontium sulfate mineral, and strontianite, a carbonate mineral. Though the vast majority of strontium compounds used are derived from the carbonate, celestite is more commonly mined, as it tends to occur more frequently in deposits large enough to exploit economically. Almost all of the strontium extracted is therefore converted from the sulfate to the carbonate. For the rare cases where strontium metal is needed, it is produced by reducing strontium oxide with aluminum.
Products
 Strontium has low tech applications as an additive to flares and pyrotechnics because of the bright crimson flame produced by its salts. It also
Strontium has low tech applications as an additive to flares and pyrotechnics because of the bright crimson flame produced by its salts. It also has many high technology applications because of its high refractive index as a titanate in glass, as a "getter" in electron tubes and as a dopant for numerous perovskite formulations to produce cathodes for oxygen generation or solid oxide fuel cells. Historically the primary use of strontium has been to produce CRT glass for color televisions and computer monitors. Strontium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Strontium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Strontium fluorides is an insoluble strontium source for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Strontium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
has many high technology applications because of its high refractive index as a titanate in glass, as a "getter" in electron tubes and as a dopant for numerous perovskite formulations to produce cathodes for oxygen generation or solid oxide fuel cells. Historically the primary use of strontium has been to produce CRT glass for color televisions and computer monitors. Strontium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Strontium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Strontium fluorides is an insoluble strontium source for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Strontium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Strontium Properties
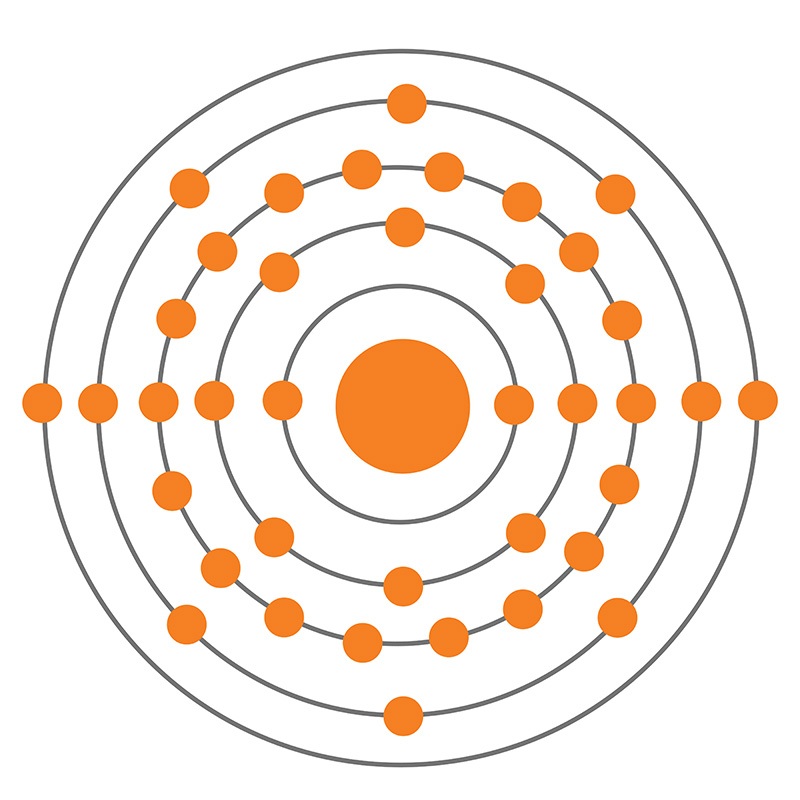
 Strontium is a Block S, Group 2, Period 5 element. The number of electrons in each of Strontium's shells is 2, 8, 18, 8, 2 and its electron configuration is [Kr] 5s2.
Strontium is a Block S, Group 2, Period 5 element. The number of electrons in each of Strontium's shells is 2, 8, 18, 8, 2 and its electron configuration is [Kr] 5s2.  Strontium was discovered by William Cruickshank in 1787 and first isolated by Humphry Davy in 1808. Strontium was named after the Scottish town it was discovered in.
Strontium was discovered by William Cruickshank in 1787 and first isolated by Humphry Davy in 1808. Strontium was named after the Scottish town it was discovered in.
Health, Safety & Transportation Information for Strontium
The non-radioactive isotopes of Strontium are not toxic. Safety data for Strontium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Strontium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H260-H315 |
| Hazard Codes | F,Xi |
| Risk Codes | 11-14-36 |
| Safety Precautions | 26 |
| RTECS Number | UN 3208 4.3/PG 1 |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Strontium Isotopes
Strontium has four stable isotopes: 84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%).
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 73Sr | 72.96597(64)# | >25 ms | ß+ to 73Rb; ß+ + p to 72kr | 1/2-# | N/A | 579.71 | - |
| 74Sr | 73.95631(54)# | 50# ms [>1.5 µs] | ß+ to 74Rb | 0+ | N/A | 596.18 | - |
| 75Sr | 74.94995(24) | 88(3) ms | ß+ to 75Rb; ß+ + p to 74kr | (3/2-) | N/A | 610.78 | - |
| 76Sr | 75.94177(4) | 7.89(7) s | ß+ to 76Rb | 0+ | N/A | 626.31 | - |
| 77Sr | 76.937945(10) | 9.0(2) s | ß+ to 77Rb; ß+ + p to 76kr | 5/2+ | N/A | 638.11 | - |
| 78Sr | 77.932180(8) | 159(8) s | ß+ to 78Rb | 0+ | N/A | 650.85 | - |
| 79Sr | 78.929708(9) | 2.25(10) min | ß+ to 79Rb | 3/2(-) | N/A | 661.73 | - |
| 80Sr | 79.924521(7) | 106.3(15) min | EC to 80Rb | 0+ | N/A | 674.46 | - |
| 81Sr | 80.923212(7) | 22.3(4) min | EC to 81Rb | 1/2- | 0.544 | 683.47 | - |
| 82Sr | 81.918402(6) | 25.36(3) d | EC to 82Rb | 0+ | N/A | 696.21 | - |
| 83Sr | 82.917557(11) | 32.41(3) h | EC to 83Rb | 7/2+ | -0.898 | 705.22 | - |
| 84Sr | 83.913425(3) | Observationally Stable | - | 0+ | N/A | 717.02 | 0.56 |
| 85Sr | 84.912933(3) | 64.853(8) d | EC to 85Rb | 9/2+ | -1.001 | 726.04 | - |
| 86Sr | 85.9092602(12) | STABLE | - | 0+ | N/A | 736.91 | 9.86 |
| 87Sr | 86.9088771(12) | STABLE | - | 9/2+ | -1.09283 | 745.92 | 7 |
| 88Sr | 87.9056121(12) | STABLE | - | 0+ | N/A | 756.79 | 82.58 |
| 89Sr | 88.9074507(12) | 50.57(3) d | ß- to 89Y | 5/2+ | -1.149 | 763.01 | - |
| 90Sr | 89.907738(3) | 28.90(3) y | ß- to 90Y | 0+ | N/A | 771.09 | - |
| 91Sr | 90.910203(5) | 9.63(5) h | ß- to 91Y | 5/2+ | -0.887 | 776.37 | - |
| 92Sr | 91.911038(4) | 2.66(4) h | ß- to 92Y | 0+ | N/A | 783.52 | - |
| 93Sr | 92.914026(8) | 7.423(24) min | ß- to 93Y | 5/2+ | N/A | 788.8 | - |
| 94Sr | 93.915361(8) | 75.3(2) s | ß- to 94Y | 0+ | N/A | 795.95 | - |
| 95Sr | 94.919359(8) | 23.90(14) s | ß- to 95Y | 1/2+ | N/A | 800.3 | - |
| 96Sr | 95.921697(29) | 1.07(1) s | ß- to 96Y | 0+ | N/A | 806.52 | - |
| 97Sr | 96.926153(21) | 429(5) ms | ß- to 97Y; ß- + n to 96Y | 1/2+ | N/A | 809.94 | - |
| 98Sr | 97.928453(28) | 0.653(2) s | ß- to 98Y; ß- + n to 97Y | 0+ | N/A | 816.15 | - |
| 99Sr | 98.93324(9) | 0.269(1) s | ß- to 99Y; ß- + n to 98Y | 3/2+ | N/A | 819.57 | - |
| 100Sr | 99.93535(14) | 202(3) ms | ß- to 100Y; ß- + n to 99Y | 0+ | N/A | 825.79 | - |
| 101Sr | 100.94052(13) | 118(3) ms | ß- to 101Y; ß- + n to 100Y | (5/2-) | N/A | 829.21 | - |
| 102Sr | 101.94302(12) | 69(6) ms | ß- to 102Y; ß- + n to 101Y | 0+ | N/A | 837.29 | - |
| 103Sr | 102.94895(54)# | 50# ms [>300 ns] | ß- to 103Y | N/A | N/A | 845.37 | - |
| 104Sr | 103.95233(75)# | 30# ms [>300 ns] | ß- to 104Y | 0+ | N/A | 844.13 | - |
| 105Sr | 104.95858(75)# | 20# ms [>300 ns] | Unknown | N/A | N/A | 852.21 | - |