About Barium
Similarly to the adjacent element cesium within its family of the alkali metals, barium is the heaviest and most reactive of the stable elements in its family, the alkaline earths. The element first gained attention in the early 17th century in Bologna, Italy, when rocks composed of barium sulfate drew alchemists due to their unique ability to emit a phosphorescent red glow for years after being burnt and exposed to sunlight. Barium’s name is derived from the alchemical term for barium sulfate "baryta," which in turn is based on the Greek term barys meaning "heavy" or "dense." Sir Humphrey Davey bestowed the name upon his discovery of the element in 1808 via the electrolysis of molten barium salts, the same technique he used to isolate the other alkali metals. Barium does not occur free in nature due to its high reactivity; it is mostly present as barium sulfate in the mineral barite (also known as barytes or heavy spar), its main commercial source. The element is also found in witherite (barium carbonate) to a lesser extent and in the fluorescent blue gemstone benitoite (barium titanium silicate), the official state gemstone of California. Natural barium is a mix of 7 different isotopes and can be produced by either electrolysis of barium chloride or the reduction of barium oxide with elemental aluminum.
Chemically similar to calcium, barium is a shiny silver metal that turns gray as its surface oxidizes in contact with air. The element is extremely electropositive and highly reactive; contact with water and alcohols causes an explosive exothermic reaction, and reactions with nonmetals such as carbon and nitrogen cause similarly exothermic reactions when heated. Because of its flammability, barium is packaged under mineral oil as a safety precaution. Additionally, soluble barium compounds are all considered extremely poisonous due to the toxicity of the Ba2+ ion and must be handled with care.
Barium has many different forms with commercially useful properties. As a metal or alloyed with aluminum, it serves as a “flashed getter” in vacuum tubes to combine with and remove residual oxygen or moisture; it can also increase the creep resistance of lead-tin alloys and enhance the structure of aluminum-silicon alloys. Barium can alloy with other metals such as zinc, lead, nickel, and tin to form intermetallic phases and alloys used as bearing alloys, deoxidizers, and (in the form of barium-nickel alloys) the basis for spark plug wires. Barium compounds such as carbonates, chlorides, oxides, hydroxides, and peroxides are used as bleaching agents, desiccants, water softeners, components of glass and ceramics, additives to oil drilling fluids, green colorings for fireworks, and rat poisons; some other applications include purification of solutions and calibration of pH equipment. Barium sulfate is uniquely insoluble in water and thus nontoxic, allowing it to be used as a radiopaque contrast media for X-ray and CAT scan imaging of the gastrointestinal tract in high purity form. The compound either by itself or in combination with zinc sulfide (a material known as lithopone) is also used as a white pigment in paints, ink, and coatings in addition to a filler for rubbers and plastics.
Some crystalline ceramic forms of barium possess unusual properties that give them specialized high technology applications. Yttrium barium copper oxide (YBCO) is a well-known high-temperature superconductor, the first material ever to be discovered that exhibits superconductivity above the temperature of liquid nitrogen (77 K). Magnetic strips of credit card and data storage devices utilize barium ferrite, a magnetic material that can take on a complex ferromagnetic fluid phase at room temperature. Barium titanate and barium zirconate (when combined, known as barium zirconate titanate or BZT) are piezoelectric, ferroelectric perovskite crystals that can function as dielectric materials in capacitors, electrolytes in solid oxide fuel cells, and nonlinear optical crystals; barium fluoride is another common optical material used in lenses, windows, and scintillators due to its wide transparency in the ultraviolet and infrared spectra.
Products
Barium has applications in glass, electronics, medicine, paints and colorants. Barium sulfate is opaque to x-rays and can be safely swallowed as a suspension, and thus  it is frequently used as a contrast medium for imaging the gastrointestinal tract. Electronic coatings based on barium titanate are essential to cell phones and other microelectronics. Barium is available in both metallic and compound forms with purities from 99% to 99.999% (ACS grade to ultra-high purity).
it is frequently used as a contrast medium for imaging the gastrointestinal tract. Electronic coatings based on barium titanate are essential to cell phones and other microelectronics. Barium is available in both metallic and compound forms with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Barium oxide is an insoluble barium source available in powder and dense pellet form for such uses as optical coating and thin film applications. Barium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Barium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Barium oxide is an insoluble barium source available in powder and dense pellet form for such uses as optical coating and thin film applications. Barium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Barium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Barium Properties
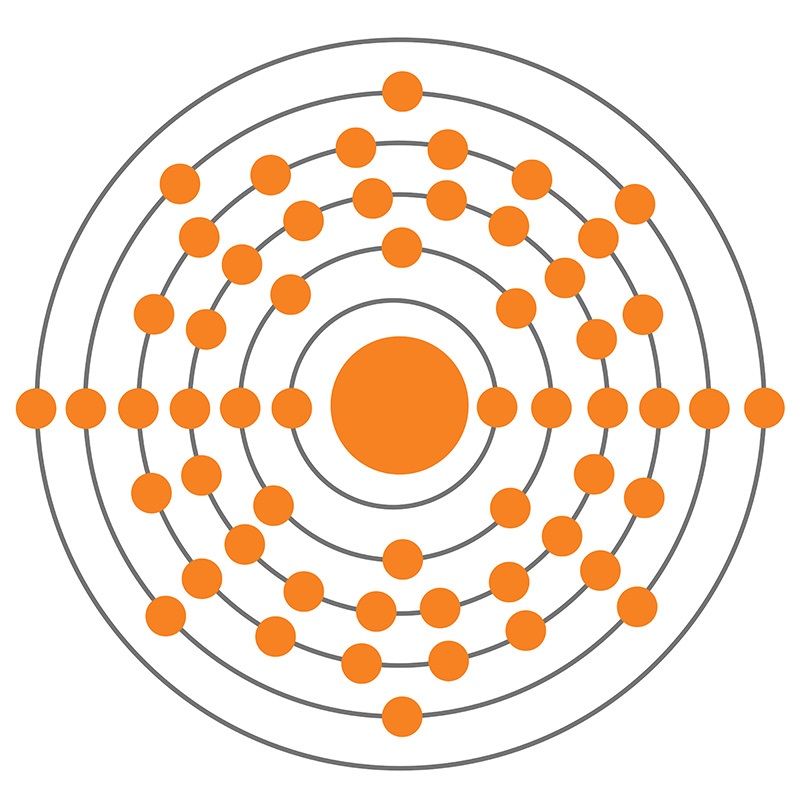
 Barium is a Block S, Group 2, Period 6 element.
Barium is a Block S, Group 2, Period 6 element. The number of electrons in each of Barium's shells is 2, 8, 18, 18, 8, 2 and its electron configuration is [Xe] 6s2.
The number of electrons in each of Barium's shells is 2, 8, 18, 18, 8, 2 and its electron configuration is [Xe] 6s2.  Barium is a member of the alkaline-earth metals. The barium atom has a radius of 222.pm and its Van der Waals radius is 268.pm. In its elemental form, CAS 7440-39-3, barium has a silvery-gray appearance. The main commercial source of barium is barite, BaSO4. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808.
Barium is a member of the alkaline-earth metals. The barium atom has a radius of 222.pm and its Van der Waals radius is 268.pm. In its elemental form, CAS 7440-39-3, barium has a silvery-gray appearance. The main commercial source of barium is barite, BaSO4. Barium was discovered by Carl Wilhelm Scheele in 1772 and first isolated by Humphry Davy in 1808.
Health, Safety & Transportation Information for Barium
Barium powder can ignite spontaneously in air, and when Barium compounds are water or acid soluble, they are very poisonous. Safety data for Barium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Barium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H261-H315-H319-H335 |
| Hazard Codes | F,Xi |
| Risk Codes | 11-14/15-36/37/38 |
| Safety Precautions | 16-26-36/37-43 |
| RTECS Number | CQ8370000 |
| Transport Information | UN 1400 4.3/PG 2 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Barium Isotopes
Barium has six stable isotopes: 132Ba, 134Ba, 135Ba, 136Ba, 137Ba, and 138Ba.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 114Ba | 113.95068(15) | 530(230) ms [0.43(+30-15) s] | β+ + p to 114Xe; β+ to 114Cs; β+ + α to 110I | 0+ | N/A | 904.72 | - |
| 115Ba | 114.94737(64)# | 0.45(5) s | β+ to 115Cs; β+ + p to 114Xe | (5/2+)# | N/A | 922.11 | - |
| 116Ba | 115.94138(43)# | 1.3(2) s | β+ to 116Cs; β+ + p to 115Xe | 0+ | N/A | 930.19 | - |
| 117Ba | 116.93850(32)# | 1.75(7) s | β+ to 117Cs; β+ + α to 113I; β+ + p to 116Xe | (3/2)(+#) | N/A | 947.59 | - |
| 118Ba | 117.93304(21)# | 5.2(2) s | β+ to 118Cs; β+ + p to 117Xe | 0+ | N/A | 955.67 | - |
| 119Ba | 118.93066(21) | 5.4(3) s | β+ to 119Cs; β+ + p to 118Xe | (5/2+) | N/A | 963.74 | - |
| 120Ba | 119.92604(32) | 24(2) s | β+ to 120Cs | 0+ | N/A | 981.14 | - |
| 121Ba | 120.92405(15) | 29.7(15) s | β+ to 121Cs; β+ + p to 120Xe | 5/2(+) | N/A | 989.22 | - |
| 122Ba | 121.91990(3) | 1.95(15) min | β+ to 122Cs | 0+ | N/A | 1006.61 | - |
| 123Ba | 122.918781(13) | 2.7(4) min | β+ to 123Cs | 5/2(+) | N/A | 1014.69 | - |
| 124Ba | 123.915094(13) | 11.0(5) min | β+ to 124Cs | 0+ | N/A | 1022.77 | - |
| 125Ba | 124.914473(12) | 3.5(4) min | β+ to 125Cs | 1/2(+#) | N/A | 1030.85 | - |
| 126Ba | 125.911250(13) | 100(2) min | β+ to 126Cs | 0+ | N/A | 1038.93 | - |
| 127Ba | 126.911094(12) | 12.7(4) min | β+ to 127Cs | 1/2+ | N/A | 1047.01 | - |
| 128Ba | 127.908318(11) | 2.43(5) d | EC to 128Cs | 0+ | N/A | 1064.4 | - |
| 129Ba | 128.908679(12) | 2.23(11) h | EC to 129Cs | 1/2+ | -0.4 | 1072.48 | - |
| 130Ba | 129.9063208(30) | 1.6 x 1021 y | 2EC to 130Xe | 0+ | N/A | 1080.56 | 0.106 |
| 131Ba | 130.906941(3) | 11.50(6) d | EC to 131Cs | 1/2+ | 0.7081 | 1088.64 | - |
| 132Ba | 131.9050613(11) | Observationally Stable | - | 0+ | N/A | 1096.72 | 0.101 |
| 133Ba | 132.9060075(11) | 10.51(5) y | EC to 133Cs | 1/2+ | 0.7717 | 1104.8 | - |
| 134Ba | 133.9045084(4) | STABLE | - | 0+ | N/A | 1112.87 | 2.417 |
| 135Ba | 134.9056886(4) | STABLE | - | 3/2+ | 0.837943 | 1120.95 | 6.592 |
| 136Ba | 135.9045759(4) | STABLE | - | 0+ | N/A | 1129.03 | 7.854 |
| 137Ba | 136.9058274(5) | STABLE | - | 3/2+ | 0.937365 | 1137.11 | 11.232 |
| 138Ba | 137.9052472(5) | STABLE | - | 0+ | N/A | 1145.19 | 71.698 |
| 139Ba | 138.9088413(5) | 83.06(28) min | β- to 139La | 7/2- | -0.97 | 1153.27 | - |
| 140Ba | 139.910605(9) | 12.752(3) d | β- to 140La | 0+ | N/A | 1152.03 | - |
| 141Ba | 140.914411(9) | 18.27(7) min | β- to 141La | 3/2- | N/A | 1160.11 | - |
| 142Ba | 141.916453(7) | 10.6(2) min | β- to 142La | 0+ | N/A | 1168.19 | - |
| 143Ba | 142.920627(14) | 14.5(3) s | β- to 143La | 5/2- | N/A | 1166.95 | - |
| 144Ba | 143.922953(14) | 11.5(2) s | β- to 144La | 0+ | N/A | 1175.03 | - |
| 145Ba | 144.92763(8) | 4.31(16) s | β- to 145La | 5/2- | N/A | 1183.11 | - |
| 146Ba | 145.93022(8) | 2.22(7) s | β- to 146La; β- + n to 145La | 0+ | N/A | 1181.87 | - |
| 147Ba | 146.93495(22)# | 0.893(1) s | β- to 147La; β- + n to 146La | (3/2+) | N/A | 1189.95 | - |
| 148Ba | 147.93772(9) | 0.612(17) s | β- to 148La; β- + n to 147La | 0+ | N/A | 1198.03 | - |
| 149Ba | 148.94258(21)# | 344(7) ms | β- to 149La; β- + n to 148La | 3/2-# | N/A | 1196.79 | - |
| 150Ba | 149.94568(43)# | 300 ms | β- to 150La | 0+ | N/A | 1204.87 | - |
| 151Ba | 150.95081(43)# | 200# ms [>300 ns] | β- to 151La | 3/2-# | N/A | 1203.63 | - |
| 152Ba | 151.95427(54)# | 100# ms | β- to 152La | 0+ | N/A | 1211.71 | - |
| 153Ba | 152.95961(86)# | 80# ms | β- to 153La | 5/2-# | N/A | 1219.79 | - |