About Nitrogen
Nitrogen is an exceedingly abundant element, both in our galaxy, where it is estimated to be the seventh most abundant element, and on earth, where it comprises nearly eighty percent of the atmosphere. It is additionally one of the major elements required for life, as a primary component of proteins and DNA. In fact, nitrogen is often the limiting element which determines whether organisms will grow successfully in a given environment. Early in the history of agriculture, farmers came to an intuitive understanding of the importance of plant nutrients in soil and developed ways to manage the fertility of their soil either by implementing crop rotation or applying animal waste as fertilizer. Though these early farmers did not understand the chemistry underlying their methods, one of the most important effects of these practices was the maintenance of sufficient nitrogen in the soil to support plant growth.
Nitrogen compounds were used more explicitly by alchemists, who knew nitric acid as aqua fortis, which they often prepared by treating niter--potassium nitrate--with sulfuric acid. Potassium nitrate was also known as saltpeter, and later found use in explosive mixtures such as gunpowder. Potassium nitrate for both alchemy and explosives was initially derived from natural sources. It could be obtained in pure form when crystals of the substance formed on cave walls, but more commonly it was derived from human or animal waste. Methods for accomplishing this varied, but almost always included a final step in which a nitrate-rich solution was treated with potassium carbonate in the form of wood ashes (potash). After the introduction of saltpeter-containing explosives, saltpeter production became so important that in many European countries, government agents had the right to confiscate soils appropriate for its production, and some even mandated that human or animal waste be saved or collected for this purpose.
Nitrogen was also a component of the common alchemist’s tool aqua regia, a mixture of nitric and hydrochloric acids notable for its ability to dissolve nearly any metal, including the noble metal gold. Though aqua regia could be prepared by treating a variety of chloride compounds with nitric acid, including common table salt, historical texts often reference the use of a mineral known as sal ammoniac--ammonium chloride, another nitrogen compound--for this purpose. The Romans collected sal ammoniac from deposits near the Temple of Amun in ancient Libya; its name literally means “salt of Amun" and serves as the basis for the modern names of all ammonium compounds. In addition to being important in alchemy, ammonium chloride came to be used as a food additive, as a flux in soldering tin, as medical treatment for various ailments, and in the preparation of other ammonium compounds. Ammonium compounds were also prepared from the fermentation of urine, used to produce dying agents used in the middle ages, and from the distillation of animal hooves and horns, lending the name “spirit of hartshorn” to aqueous solutions of ammonia and “salt of hartshorn” to ammonium carbonate. Ammonium carbonate’s medical use as smelling salts dates back to ancient Rome, and the compound later came into use as a leavening agent in baking.
Despite these widespread historical applications of nitrogen compounds, the element itself was discovered relatively late in human history. In the mid-eighteenth century, common gaseous compounds were just beginning to gain the attention of chemists. Initially, it was merely recognized that air had both components that would support breathing of living creatures and components that would suffocate them. Dr. Joseph Black had studied the first of these suffocating gases: ”fixed air”, or what modern science knows as carbon dioxide. Shortly thereafter, Henry Cavendish showed in experiments that another component of air also did not support life, but was chemically distinct from “fixed air”, but he did not publish his findings. In 1772, Daniel Rutherford, Dr. Black’s student, isolated this same substance and published his findings regarding the substance which he called “phlogisticated air”. The famous chemist Antoine Lavoisier called this gas “azote” from the Greek azotos meaning “lifeless”. This name was retained in some languages, and is the basis of the English names of some nitrogen compounds such as azides. The modern English name for the element comes from the association of the gas with nitric acid, which was known to be derived from niter.
Coincidentally, at this time, other scientists were beginning investigations into the chemistry of plant nutrition. Europe’s population was booming, and concerns about this growth outpacing the capacity for food production, particularly as highlighted by Thomas Malthus’s “Essay on Population, had helped produce an intense focus on increasing agricultural productivity. By the early nineteenth century, the importance of nitrogen content in fertilizer was recognized, and demand for fertilizers rich in nitrates began to boom. Relatively few large deposits of nitrate minerals exist, but one such source was found in South America: the salt flats of the Atacama Desert. By 1830, sodium nitrate was exported from South America to Europe for use as fertilizer, as was guano, a source of both nitrates and of phosphorus, another essential plant nutrient. Chemists also determined a way to convert the Atacaman nitrate salts into quality saltpeter for use in gunpowder and explosives, which further drove demand for this resource.
Though small initially, this trade grew rapidly, and soon grew contentious--in 1879, Bolivia, Peru, and Chile went to war for control of the Atacama desert and its associated saltpeter trade. Europe’s intense and ever-increasing demand for saltpeter, in addition to its heavy reliance on South America for its supply, prompted increasingly urgent discussion of what came to be known as “The Nitrogen Problem”. It was quickly recognized that atmospheric nitrogen was potential solution to this problem--a nearly limitless potential source of nitrates for fertilizer and explosives--but that it needed to be “fixed” into forms that could be used by plants. Thus, a race began to devise some chemical means of achieving nitrogen fixation.
This chemical quest was enormously difficult; in its pure form as a diatomic gas, nitrogen exhibits a profound chemical stability due to the strength of the triple bond uniting the two atoms, a configuration that requires enormous energy inputs to disturb. Early chemical processes inefficiently produced nitrous oxides using massive amounts of electricity, and were used industrially only briefly. The real breakthrough came from a German chemist named Fritz Haber, who developed a process that produced ammonia from hydrogen and nitrogen gases at high temperatures and pressures. Haber’s initial process required expensive catalysts, but another chemist, Bosch, developed a more economically viable iron-based catalyst, and scaled up the process for industrial production. The Haber-Bosch process, as it came to be known, is still quite energy intensive, but was a major advance over alternatives and allowed chemically-fixed nitrogen fertilizers to come into widespread use. The use of these fertilizers is said to have saved millions from starvation, and Fritz Haber was awarded the 1918 Nobel Prize in Chemistry for his role in what has since been called the most important industrial process ever developed.
However, the plentiful fixed nitrogen derived from the Haber-Bosch process was not all used for fertilizer. It could also be easily used to produce saltpeter for gunpowder, as well as potent nitrogen-based explosives. Both nitroglycerine (the explosive component of dynamite) and trinitrotoluene (TNT) had both been initially synthesized in the mid-nineteenth century, but could now be manufactured and used in warfare at unprecedented rates. Nitrogen compounds have retained dominance in explosive applications into modern day: plastic explosives such as C-4 all require explosive nitrogen compounds. Beyond their obvious military utility, nitrogen explosives are also important in mining, and as propellants in devices such as automobile airbags. Unfortunately, the historic link between nitrogen fertilizers and the malicious use of explosives remains salient, as improvised explosives can be produced easily from common fertilizers such as ammonium nitrate. The significant threat imposed by nitrogen explosives has lead to strict regulation of many such nitrogen compounds.
Nitrogen finds many applications beyond the fields of agriculture and warfare. Nitrogen gas, purified from air through fractional distillation of liquid air, pressure swing adsorption, or membrane separation, is used widely in industry, serving as filler gas in food packaging, a working atmosphere in the manufacture of delicate electronics, and in dozens of other settings where a fairly inert environment is required. Liquid nitrogen is equally ubiquitous as a relatively inexpensive means to maintain temperatures far below the freezing point of water, which is used in many industrial processes, cryopreservation of biological tissues, and even in the production of novel food products in molecular gastronomy. Nitrous and nitric acids continue to be important reagents in analytical and industrial chemistry, and the simple nitrogen compound ammonia is important as a solvent and antiseptic frequently found in cleaning products, and in many niche applications.
Many additional classes of nitrogen compounds also have important uses. A nitrogen atom connected to a carbon atom with a triple bond is known as a cyano group, and both inorganic and organic compounds containing this group, known respectively as cyanides and nitriles, have important applications. Most cyanides are highly toxic due to the ability of the CN- ion to inhibit enzymes necessary for aerobic respiration, but they are nonetheless important for the cyanide process for mining gold, as well as in the industrial production of nitriles. Commercially useful nitrile compounds include cyanoacrylates, strong adhesives known under trade names such as Super Glue, and various polyacrylonitrile materials. The latter class includes nitrile rubber, used in non-latex disposable gloves, acrylic fiber, used in textiles and as a precursor to carbon fiber, and various copolymer plastics.
Nitrogen is in fact a key component of many important synthetic materials. Polyacrylamide materials absorb water, allowing them to act as water-soluble thickeners, flocculants and as soil conditioners that increase aeration and reduce water run-off. In gel form, they additionally are important in electrophoresis methods in biological research and in soft contact lenses. Celluloid, a nitrated cellulose polymer, was once used for standard photographic film, but its use was discontinued due to fires associated with its extreme flammability. Polyamides both occur naturally, as proteins, and as ubiquitous synthetic fibers such as kevlar and nylon, all of which owe their durability and strength of their amide linkages, which form from between carboxylic acids and nitrogen-containing amine groups. Finally, polymers called polyphosphazenes, which have backbones composed of phosphorus and nitrogen, are used in many specialty applications due to their durability and ability to be fabricated to produce useful properties such as fluorescence.
Many synthetic small-molecule nitrogen compounds are also important industrial products. Azo compounds, which contain the functional group R-N=N-R', are important dyes and pigments. They are found in such basic pigment applications as textile dying, artist paints, and industrial coloring agents, where they are particularly important due to exhibiting less toxicity compared to alternative metal-based pigments. Some also have more technical applications, serving as recording layer of CD-R and DVD-R optical discs, as the sunlight-absorbing compounds in dye-sensitized solar cells, and as pH indicators. Additionally, many biological agents, both pharmaceuticals and poisons such as pesticides, are synthetic nitrogen compounds. Amine, amide, and triazole compounds often serve such purposes by closely mimicking the structure of a natural biological compound in order to replicate or block that compound’s natural function, while the extremely stable cyano group is often added to drugs to increase their half-lives in the body. Hydrazine, a simple nitrogen compound derived from ammonia, is important in the synthesis of many of these small molecules and nitrogen polymer precursors, and additionally is an important aerospace fuel. Nitrous oxide, popularly known for its analgesic effect exploited in its use as the mild analgesic laughing gas, is another important ammonia derivative. It is used as an oxidizer in rocket motors and combustion engines, and additionally as an aerosol propellant.
Finally, nitrides, simple compounds of nitrogen and a metallic or metalloid element, are important commercial materials. Silicon and boron nitrides are extremely hard ceramics used as refractory materials, cutting materials, hard coatings, and as a component of composite materials. Silicon nitride is additionally used in orthopedic implants, for extremely durable and low-friction bearings, and as an insulator or etchant mask in integrated circuit manufacturing. Boron nitride is useful as a lubricant that remains stable under extreme conditions, and in nanomaterials being investigated for applications in chemical catalysis, computing devices, and aerospace applications. Lithium nitride can store large volumes of hydrogen gas, and is under investigation for use in hydrogen-based alternative energy solutions. Finally, gallium nitride is a wide-bandgap semiconductor material important for the production of light-emitting diodes (LEDs).
Nitrogen Properties
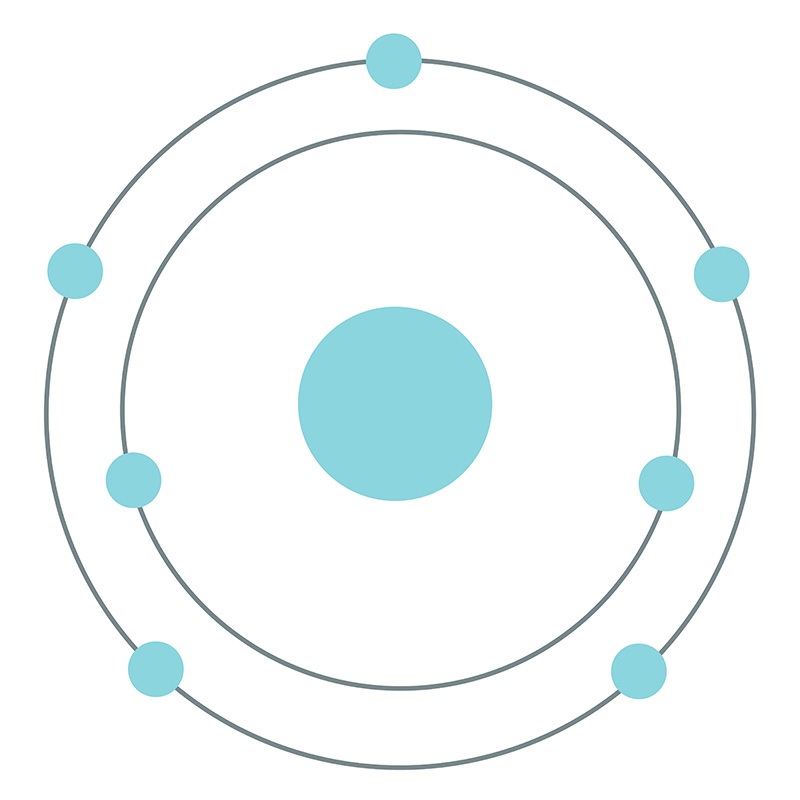
![]() Nitrogen is a Block P, Group 15, Period 2 element. Its electron configuration is [He]2s22p3. In its elemental form, nitrogen's CAS number is 7727-37-9. Nitrogen is an odorless, tasteless, colorless and mostly inert gas. It is the seventh most abundant element in the universe and it constitutes 78.09% (by volume) of Earth's atmosphere. Nitrogen was discovered by Daniel Rutherford in 1772. Nitrogen information, including technical data, safety data, properties, research, applications and other useful facts are specified below. Scientific facts such as the atomic structure, ionization energy, abundance on Earth, conductivity and thermal properties are included.
Nitrogen is a Block P, Group 15, Period 2 element. Its electron configuration is [He]2s22p3. In its elemental form, nitrogen's CAS number is 7727-37-9. Nitrogen is an odorless, tasteless, colorless and mostly inert gas. It is the seventh most abundant element in the universe and it constitutes 78.09% (by volume) of Earth's atmosphere. Nitrogen was discovered by Daniel Rutherford in 1772. Nitrogen information, including technical data, safety data, properties, research, applications and other useful facts are specified below. Scientific facts such as the atomic structure, ionization energy, abundance on Earth, conductivity and thermal properties are included.
Health, Safety & Transportation Information for Nitrogen
The below information applies to elemental Nitrogen.
| Safety Data | |
|---|---|
| Material Safety Data Sheet | MSDS |
| Signal Word | Warning |
| Hazard Statements | H280 |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | UN 1066 2.2 |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Nitrogen Isotopes
Nitrogen has two stable isotopes: 14N and 15N.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 10N | 10.04165(43) | 200(140)×10-24 s [2.3(16) ] | p to 9C | (2-) | N/A | 34.74 | - |
| 11N | 11.02609(5) | 590(210)×10-24 s [1.58(+75-52) ] | p to 10C | 1/2+ | N/A | 56.79 | - |
| 12N | 12.0186132(11) | 11.000(16) ms | EC to 12C; EC + 3a to n | 1+ | 0.457 | 72.32 | - |
| 13N | 13.00573861(29) | 9.965(4) min | EC to 13C | 1/2- | 0.3222 | 92.51 | - |
| 14N | 14.0030740048(6) | STABLE | - | 1+ | 0.4037607 | 102.45 | 99.632 |
| 15N | 15.0001088982(7) | STABLE | - | 1/2- | -0.2831892 | 113.33 | 0.368 |
| 16N | 16.0061017(28) | 7.13(2) s | ß- to 16O | 2- | N/A | 115.82 | - |
| 17N | 17.008450(16) | 4.173(4) s | ß- to 17O; ß- + n to 16O | 1/2- | N/A | 122.03 | - |
| 18N | 18.014079(20) | 622(9) ms | ß- to 18O; ß- + a to 14C | 1- | N/A | 124.52 | - |
| 19N | 19.017029(18) | 271(8) ms | ß- to 19O | (1/2)- | N/A | 129.8 | - |
| 20N | 20.02337(6) | 130(7) ms | ß- to 20O | N/A | N/A | 132.29 | - |
| 21N | 21.02711(10) | 87(6) ms | ß- to 21O | 1/2-# | N/A | 136.64 | - |
| 22N | 22.03439(21) | 13.9(14) ms | ß- to 22O | N/A | N/A | 138.2 | - |
| 23N | 23.04122(32)# | 14.5(24) ms [14.1(+12-15) ms] | ß- to 23O | 1/2-# | N/A | 139.76 | - |
| 24N | 24.05104(43)# | <52 ns | n to 23N | N/A | N/A | 138.52 | - |
| 25N | 25.06066(54)# | <260 ns | Unknown | 1/2-# | N/A | 138.22 | - |