SECTION 1. IDENTIFICATION
Product Name: Cadmium Chloride Monohydrate
Product Number: All applicable American Elements product codes, e.g. CD-CL-018-C.1HYD
CAS #: 35658-65-2
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
2.1. Classification of the substance or mixture
Classification EC 67/548 or EC 1999/45
Classification : T+; R26
T; R25-R48/23/25
N; R50-53
Hazard Class and Category Code(s), Regulation (EC) No 1272/2008 (CLP)
Health hazards : Specific Target Organ Toxicity - Repeated exposure - Category 1 - Danger (CLP :
STOT RE 1) H372
Acute toxicity, Oral - Category 3 - Danger (CLP : Acute Tox. 3) H301
Acute toxicity, Inhalation - Category 2 - Danger (CLP : Acute Tox. 2) H330
Environmental hazards : Hazardous to the aquatic environment - Acute hazard - Category 1 - Warning (CLP
: Aquatic Acute 1) H400
Hazardous to the aquatic environment - Chronic hazard - Category 1 - Warning (
CLP : Aquatic Chronic 1) H410
2.2. Label elements
Labelling EC 67/548 or EC 1999/45
Symbol(s) êT êN
Symbol(s) : T+ : Very toxic
N : Dangerous for the environment
R Phrase(s) : R45 : May cause cancer.
R46 : May cause heritable genetic damage.
R60 : May impair fertility.
R61 : May cause harm to the unborn child.
R25 : Toxic if swallowed.
R26 : Very toxic by inhalation.
R48/23/25 : Toxic : danger of serious damage to health by prolonged exposure
through inhalation and if swallowed.
R50/53 : Very toxic to aquatic organisms, may cause long-term adverse effects in
the aquatic environment.
S Phrase(s) : S53 : Avoid exposure - obtain special instructions before use.
S45 : In case of accident or if you feel unwell, seek medical advice immediately (
show the label when possible).
S60 : This material and its container must be disposed of as hazardous waste.
S61 : Avoid release to the environment. Refer to special instructions/Safety data
sheets.
Labelling Regulation EC 1272/2008 (CLP)
Hazard pictograms MÁ M“ MÅ
Signal words : Danger
Hazard statements : H330 : Fatal if inhaled.
H301 : Toxic if swallowed.
H372 : Causes damage to organs through prolonged or repeated exposure.
H410 : Very toxic to aquatic life with long lasting effects.
Precautionary statements
• Prevention : P284: Wear respiratory protection.
P271: Use only outdoors or in a well-ventilated area.
P260: Do not breathe dust, fume, gas, mist, Vapors, spray.
P273: Avoid release to the environment.
P264: Wash thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
• Response : P301+P310: IF SWALLOWED : Immediately call a POISON CENTER or doctor.
P391: Collect spillage.
P304+P340: IF INHALED : Remove to fresh air and keep at rest in a position
comfortable for breathing.
P314: Get medical advice if you feel unwell.
• Storage : P403+P233: Store in well-ventilated place. Keep container tightly closed.
P405: Store locked up.
• Disposal considerations : P501: Dispose of this material and its container to hazardous or special waste
collection point, in accordance with local, regional, national and/or international
regulation.
2.3. Other hazards
Other hazards : The substance does not fulfil the criteria to be identified as PBT substance or vPvB
substance according to Annex XIII of Regulation REACH.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance / Preparation : CADMIUM CHLORIDE (MONOHYDRATE) 98%
CAS No :35658-65-2
Substance.
Contains no other components or impurities which will influence the classification of the product.
SECTION 4. FIRST AID MEASURES
4.1. Description of first aid measures
Inhalation : Assure fresh air breathing. Allow the victim to rest. Remove to fresh air and keep at
rest in a position comfortable for breathing. Immediately call a POISON CENTER or
doctor. Specific treatment is urgent (see on this label).
Skin contact : Remove affected clothing and wash all exposed skin area with mild soap and
water, followed by warm water rinse.
Eye contact : Rinse immediately with plenty of water. Obtain medical attention if pain, blinking or
redness persist.
Ingestion : Do NOT induce vomiting. Rinse mouth. Immediately call a POISON CENTER or
doctor. Specific treatment (see on this label).
4.2. Most important symptoms and effects, both acute and delayed
Symptoms relating to use : Causes damage to organs through prolonged or repeated exposure.
Fatal if inhaled.
Swallowing a small quantity of this material will result in serious health hazard.
4.3. Indication of any immediate medical attention and special treatment needed
General information : Never give anything by mouth to an unconscious person. If you feel unwell, seek
medical advice (show the label where possible).
SECTION 5. FIREFIGHTING MEASURES
5.1. Extinguishing media
Suitable extinguishing media : Foam. Dry powder. Carbon dioxide. Water spray. Sand.
Unsuitable extinguishing media : Do not use a heavy water stream.
Surrounding fires : Use water spray or fog for cooling exposed containers.
5.2. Special hazards arising from the substance or mixture
Hazardous combustion products : Under fire conditions, hazardous fumes will be present.
5.3. Advice for fire-fighters
Protection against fire : Do not enter fire area without proper protective equipment, including respiratory
protection.
Special procedures : Exercise caution when fighting any chemical fire. Avoid (reject) fire-fighting water to
enter environment.
SECTION 6. ACCIDENTAL RELEASE MEASURES
6.1. Personal precautions, protective equipment and emergency procedures
For emergency responders : Equip cleanup crew with proper protection.
Ventilate area.
For non-emergency personnel : Evacuate unnecessary personnel.
6.2. Environmental precautions
Environmental precautions : Prevent entry to sewers and public waters. Notify authorities if product enters
sewers or public waters.
Avoid release to the environment.
6.3. Methods and material for containment and cleaning up
Clean up methods : On land, sweep or shovel into suitable containers. Minimize generation of dust.
Store away from other materials.
6.4. Reference to other sections
See section 8. Exposure controls/personal protection
SECTION 7. HANDLING AND STORAGE
7.1. Precautions for safe handling
Handling : Use only outdoors or in a well-ventilated area. Do not eat, drink or smoke when
using this product. Wash thoroughly after handling. Do not eat, drink or smoke
when using this product. Wash thoroughly after handling. Do not breathe dust,
fume, gas, mist, Vapors, spray.
Technical protective measures : Provide good ventilation in process area to prevent formation of Vapor.
7.2. Conditions for safe storage, including any incompatibilities : Keep only in the original container in a cool, well ventilated place. Keep container
tightly closed.
Storage - away from : Strong bases. Strong acids. Sources of ignition. Direct sunlight.
7.3. Specific end use(s)
Specific end use(s) : None.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
8.1. Exposure controls
Personal protection : Avoid all unnecessary exposure.
• Respiratory protection : Wear respiratory protection.
• Hand protection : Wear protective gloves.
• Eye protection : Chemical goggles or safety glasses.
• Others : When using, do not eat, drink or smoke.
8.2. Control parameters
Occupational Exposure Limits : No data available.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
9.1. Information on basic physical and chemical properties
Physical state at 20 °C : Solid.
Colour : White powder
Odor : Odorless
Odor threshold : No data available.
pH value : N/A
Melting point [°C] : No data available.
Decomposition point [°C] : N/A
Critical temperature [°C] : N/A
Auto-ignition temperature [°C] : N/A
Flammability (solid, gas) : N/A
Flash point [°C] : N/A
Boiling point [°C] : N/A
Initial boiling point [°C] : N/A
Final boiling point [°C] : N/A
Evaporation rate : N/A
Vapor pressure [20°C] : N/A
Vapor pressure mm/Hg : N/A
Vapor density : N/A
Density [g/cm3] : 3,33
Relative density, gas (air=1) : N/A
Relative density, liquid (water=1) : N/A
Solubility in water [% weight] : Freely soluble
Solubility in water : N/A
Log Pow octanol / water at 20°C : No data available.
Solubility : N/A
Viscosity at 40°C [mm2/s] : N/A
9.2. Other information
Explosive properties : N/A
Explosion limits - upper [%] : N/A
Explosion limits - lower [%] : N/A
Oxidising properties : No data available.
SECTION 10. STABILITY AND REACTIVITY
10.1. Reactivity
Reactivity : Not established.
10.2. Chemical stability
Chemical stability : Stable under recommended storage conditions.
10.3. Possibility of hazardous reactions
Hazardous reactions : Not established.
10.4. Conditions to avoid
Conditions to avoid : Direct sunlight. Extremely high or low temperatures.
10.5. Incompatible materials
Materials to avoid : Strong acids. Strong bases.
10.6. Hazardous decomposition products
Hazardous decomposition products : Fumes. Carbon monoxide. Carbon dioxide.
SECTION 11. TOXICOLOGICAL INFORMATION
11.1. Information on toxicological effects
Acute toxicity
• Inhalation : Fatal if inhaled.
• Dermal : Based on available data, the classification criteria are not met.
• Ingestion : Toxic if swallowed.
Corrosion : Based on available data, the classification criteria are not met.
Irritation : Based on available data, the classification criteria are not met.
Sensitization : Based on available data, the classification criteria are not met.
Mutagenicity : Based on available data, the classification criteria are not met.
Carcinogenicity : Based on available data, the classification criteria are not met.
Toxic for reproduction : Based on available data, the classification criteria are not met.
STOT-single exposure : Based on available data, the classification criteria are not met.
STOT-repeated exposure : Causes damage to organs through prolonged or repeated exposure.
Aspiration hazard : Based on available data, the classification criteria are not met.
SECTION 12. ECOLOGICAL INFORMATION
12.1. Toxicity
Toxicity information : Very toxic to aquatic life with long lasting effects.
12.2. Persistence - degradability
Persistence - degradability : May cause long-term adverse effects in the environment.
12.3. Bioaccumulative potential
Bioaccumulative potential : Not established.
12.4. Mobility in soil
Mobility in soil : Not established.
12.5. Results of PBT and vPvB assessment
Results of PBT and vPvB assessment : The substance does not fulfil the criteria to be identified as PBT substance or vPvB
substance according to Annex XIII of Regulation REACH.
12.6. Other adverse effects
Environmental precautions : Avoid release to the environment.
SECTION 13. DISPOSAL CONSIDERATIONS
13.1. Waste treatment methods
General : Avoid release to the environment. Dispose in a safe manner in accordance with
local/national regulations.
Dispose of this material and its container to hazardous or special waste collection
point, in accordance with local, regional, national and/or international regulation.
Special precautions : Hazardous waste due to toxicity.
SECTION 14. TRANSPORT INFORMATION
14.1. Land transport (ADR-RID)
Proper shipping name : CADMIUM COMPOUND
UN N° : 2570
H.I. nr : 60
ADR - Class : 6.1
Labelling - Transport : 6.1 : Toxic substances.
ADR - Classification code : T5
ADR - Group : III
ADR - Packing instructions : P002 R001 LP002
ADR - Limited Quantity : 5 KG
ADR - Tunnel code ’“»•‘”
: E : Passage forbidden through tunnels of category E.
14.2. Sea transport (IMDG) [English only]
Proper shipping name : CADMIUM COMPOUND
UN N° : 2570
IMO-IMDG - Class or division : 6.1 : Toxic substances.
IMO-IMDG - Packing group : III
IMO- IMDG - Packing instructions : P002 LP02
IMO-IMDG - Limited quantities : 5 kg
IMO-IMDG - Marine pollution : Yes
EMS-Nr : F-A S-A
14.3. Air transport (ICAO-IATA) [English only]
Proper shipping name : CADMIUM COMPOUND
UN N° : 2570
IATA - Class or division : 6.1 : Toxic substances.
IATA - Packing group : III
IATA - Passenger and Cargo Aircraft : ALLOWED
- Passenger and Cargo - Packing : 670
instruction
- Passenger and Cargo - Maximum : 100 kg
Quantity/Packing
IATA - Cargo only : ALLOWED
- Cargo only - Packing instruction : 677
- Cargo only - Maximum Quantity/ : 200 kg
Packing
IATA - Limited Quantites : 10 kg
ERG-Nr : 6L
SECTION 15. REGULATORY INFORMATION
15.1. Safety, health and environmental regulations/legislation specific for the substance or mixture
Safety, health and environmental : Ensure all national/local regulations are observed.
regulations/legislation specific for the
substance or mixture
REACH Restrictions - Annex XVII : The components of this product are not subject to restrictions.
REACH Authorisation - Annex XIV : The components of this product are not subject to authorization.
15.2. Chemical Safety Assessment
Chemical Safety Assessment : It has not been carried out.
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.

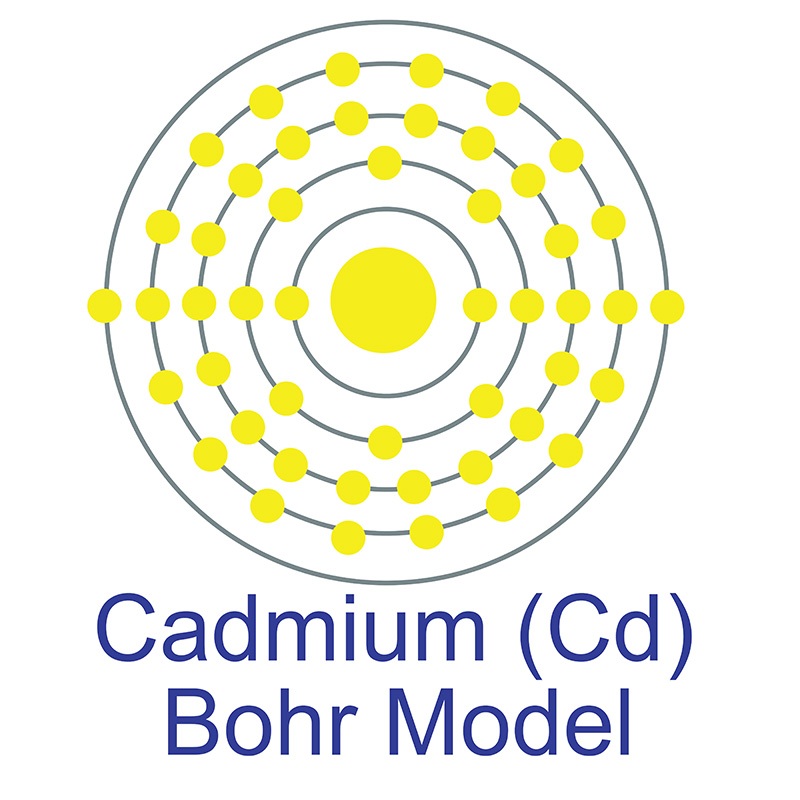 The number of electrons in each of Cadmium's shells is 2, 8, 18, 18, 2 and its electron configuration is [Kr]4d10 5s2. The cadmium atom has a radius of 151 pm and a Van der Waals radius of 230 pm. Cadmium was discovered and first isolated by Karl Samuel Leberecht Hermann and Friedrich Stromeyer in 1817. In its elemental form, cadmium has a silvery bluish gray metallic appearance. Cadmium makes up about 0.1 ppm of the earth's crust.
The number of electrons in each of Cadmium's shells is 2, 8, 18, 18, 2 and its electron configuration is [Kr]4d10 5s2. The cadmium atom has a radius of 151 pm and a Van der Waals radius of 230 pm. Cadmium was discovered and first isolated by Karl Samuel Leberecht Hermann and Friedrich Stromeyer in 1817. In its elemental form, cadmium has a silvery bluish gray metallic appearance. Cadmium makes up about 0.1 ppm of the earth's crust.  No significant deposits of cadmium containing ores are known, however, it is sometimes found in its metallic form. It is a common impurity in
No significant deposits of cadmium containing ores are known, however, it is sometimes found in its metallic form. It is a common impurity in 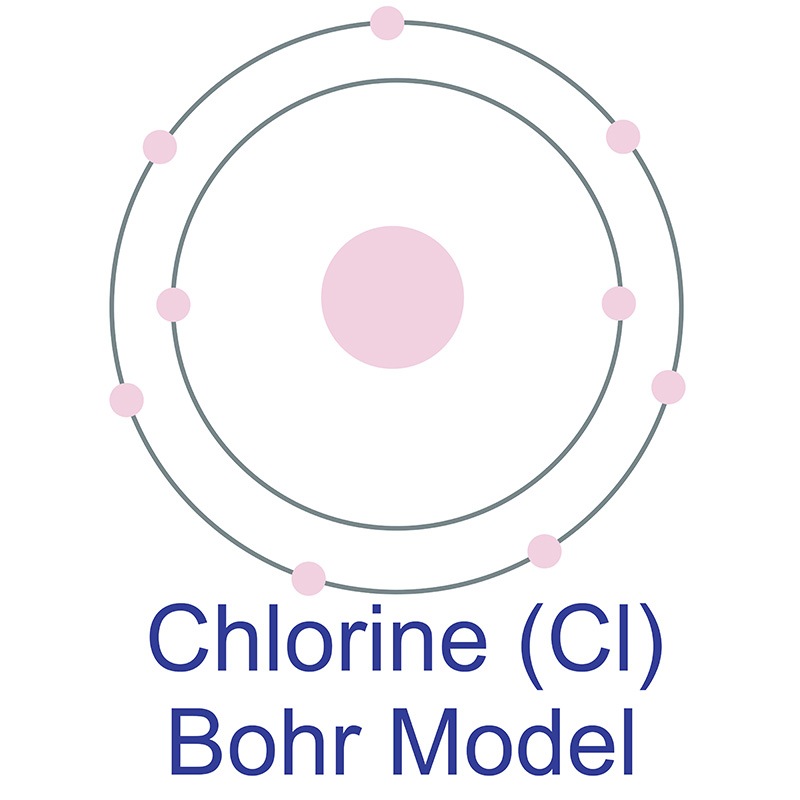 In its elemental form, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine. It has the third highest electronegativity and the highest electron affinity of all elements, making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808.
In its elemental form, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine. It has the third highest electronegativity and the highest electron affinity of all elements, making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808.