About Chlorine
Chlorine in the form of hydrochloric acid has a long history of use; as a component of aqua regia, it was used by alchemists in their experiments as early as the fourteenth century. Pure hydrochloric acid, however, was not produced until centuries later, and its chemical constituents were not known. In 1774, Carl Wilhelm Scheele was the first to document the production of a yellow-green corrosive gas, now known to be elemental chlorine, from a reaction of hydrochloric acid with magnesium oxide. However, it was a common view in chemistry at the time that all acids must contain oxygen, which initially led to the conclusion that this acid-like gas must be a compound. Despite the poor understanding its chemistry, chlorine gas quickly found practical use: in 1785, Claude Berthollet began using it to bleach textiles, shortly thereafter more convenient bleaching agents, calcium and sodium hypochlorites, came into widespread use as textile bleaches and disinfectants. Science finally came to understand the elemental nature of chlorine gas in 1810, thanks to the experimental work of Sir Humphry Davy, who named the element from the Greek chloros in reference to the distinctive color of its gaseous phase.
Chlorine continued to find additional applications throughout the eighteenth century. The photosensitivity of silver halides, including silver chloride, was widely exploited for the production of photographic images starting in 1839, and the organochlorine compound chloroform was first used as an anesthetic in 1847. In 1892, the introduction of the chloralkali process allowed the first production of chlorine on an industrial scale, an advancement which allowed for even more widespread use of chlorine in bleaches, antiseptics, and photography--all applications which continue today--as well as for expanded use of chlorine in industry.
Millions of tons of chlorine are produced and used each year, a fact that reflects the enormous importance of chlorine in modern industry. A large percentage of this chlorine is used directly in the production of polyvinyl chloride (PVC), a versatile and ubiquitous plastic used in everything from water pipes to clothing. Additionally, a significant amount of chlorine is processed to hydrochloric acid, a workhorse of an industrial chemical that finds direct use in steel production, desulfurizing petroleum products, modifying pH in oil wells, coagulating latex, and in many forms of food processing, including the refining of sugar.
Hydrochloric acid is also used to produce many other important chlorine chemicals, including metal chlorides and chlorosilanes. Metal chlorides have many uses: nickel chloride is used for nickel electroplating, zinc chlorides are used for galvanizing and as an electrolyte material in certain types of batteries, iron chloride is used in water treatment, hydrated aluminum chlorides are used in deodorants, iron and aluminum chlorides are important catalysts in organic synthesis, and several metal pentachlorides are used for chemical vapor deposition of their constituent metals in the form of thin films. Chlorosilanes are essential for the production of high purity silicon used in the semiconductor industry in the production of silicones.
Organochlorine compounds may be produced using a variety of chlorine-containing reagents and have a vast range of functions. Low molecular weight chlorinated hydrocarbons such as chloromethanes and tetrachloroethylene are vital non-polar solvents, used in applications such as degreasing and dry cleaning. A number of important herbicides and detergents are also organochlorine compounds. Organochlorine intermediates are used in the production of many types of polymers, including polycarbonates, polyurethanes, silicones, and polytetrafluoroethylene. Additionally, a significant percentage of pharmaceutical ingredients are organic compounds containing chlorine.
Chlorofluorocarbons (CFCs) and polychlorinated biphenyls (PCB) are classes of organochlorine compounds that were once found in a broad range of products, but which have since been largely phased out of use due to the discovery of their potential for damage to health and the environment. CFCs were used as refrigeration fluids, propellants in aerosols, and as solvents, but unfortunately were found to cause considerable damage to the earth’s ozone layer. They have since been replaced in most applications by the much less damaging hydrofluorocarbons (HFCs). PCBs are known to cause both acute symptoms from large exposures and cancer when they accumulate in the body over time. Once used as plasticizers, fire retardants, and coolants, and found in adhesives and paints, PCBs have now been completely banned in some countries, while others have significantly limited their use.
Chlorine’s relationship to biological organisms is somewhat paradoxical. Chlorine ions, usually obtained in the form of sodium chloride--table salt--are absolutely necessary for life: the human body uses them to maintain pH and electrical charge balances in body fluids, makes hydrochloric acid to breakdown food in the stomach, and even produces hypochlorite (chlorine bleach) compounds to help destroy infectious agents. However, chlorine in the form of gas, concentrated hydrochloric acid, or many other chlorine compounds is quite toxic. Chlorine gas has been used as a chemical weapon, and many other chemical warfare agents are chlorine compounds.
Additionally, many organochlorine compounds are both toxic and known to persist for long periods in the environment. For this reason, many such compounds have been either withdrawn from use entirely or tightly regulated. The United Nations Environmental Program produced a list of twelve “dirty dozen” persistent organic pollutants (POPs) of particular concern in 2001; all of which are chlorinated organic compounds. However, it must be noted that the problems with many chlorine compounds--their toxicity and tendency to persist in biological systems--are intimately tied to the advantages of using chlorine in many applications. In pharmaceutical manufacturing, carbon-chlorine bonds enable a drug to remain intact and active in the body for longer periods, while chlorine disinfectants directly exploit the reactivity and resultant toxicity of some chlorine products. It is therefore vital that use of chlorine products be managed responsibly, as their associated risks can never be completely eliminated.
The most common natural chlorine compound, sodium chloride, maybe be mined as rock salt or produced from evaporated saltwater. Much rock salt is used directly for deicing roads and sidewalks, and additionally some salt is simply processed to suitable forms for use in food. The rest is processed using the chloralkali process, producing both chlorine gas and sodium hydroxide through the electrolysis of brine. Very pure hydrochloric acid is produced by reacting chlorine and hydrogen gases. Additionally, hydrochloric acid is produced as a byproduct of many organic synthesis reactions, and therefore a significant quantity of technical and industrial grade hydrochloric acid are recovered from industrial chemical processes.
Products
- (1R,2R)-(-)-[1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicyl-idene)]aluminum(III) Chloride
- (1S,2S)-(+)-[1,2-Cyclohexanediamino-N,N'-bis(3,5-di-t-butylsalicyl-idene)]aluminum(III) Chloride
- (Bicyclo[2.2.1]hepta-2,5-diene)[(2S,3S)-bis (diphenylphosphino)butane]rhodium(I) Perchlorate
- (Bicyclo[2.2.1]hepta-2,5-diene)[1,1'-bis (diphenylphosphino)ferrocene]rhodium(I) Perchlorate
- (Triisopropylsiloxy)methyl Chloride
- (Trimethylsilyl)methylmagnesium Chloride Solution
- 1,1-Dimethylpropylmagnesium Chloride Solution
- 1,1'-Dipropylhafnocene Dichloride
- 1,2-Bis(diphenylphosphino)ethane Nickel(II) Chloride
- 1-Ethyl-3-methylimidazolium chloride-aluminum chloride
- 2-(Trimethylsilyl)ethanesulfonyl chloride
- 2,2,6,6-Tetramethylpiperidinylmagnesium Chloride Lithium Chloride Complex Solution
- 3-cyclohexyl Magnesium Chloride
- Allylmagnesium Chloride
- Aluminum 1,8,15,22-tetrakis(phenylthio)-29H,31H-phthalocyanine Chloride
- Aluminum 1,8,15,22-tetraphenoxy-29H,31H-phthalocyanine Chloride
- Aluminum 2,3-naphthalocyanine Chloride
- Aluminum 2,9,16,23-tetrakis(phenylthio)-29H,31H-phthalocyanine Chloride
- Aluminum Chloride Tetrahydrofuran Complex
- Aluminum Phthalocyanine Chloride
- Benzylmagnesium Chloride
- Bis(2-methylindenyl)zirconium Dichloride
- Bis(cyclopentadienyl)molybdenum(IV) Dichloride
- Bis(ethylcyclopentadienyl)niobium(IV) Dichloride
- Bis(ethylcyclopentadienyl)zirconium Dichloride
- Bis(isopropylcyclopentadienyl)titanium Dichloride
- Bis(pentamethylcyclopentadienyl)hafnium Dichloride
- Bis(tricyclohexylphosphine)nickel(II) Dichloride
- Bis(triphenylphosphoranylidene)ammonium Chloride
- Bis[(2-dimethylamino)phenyl]amine Nickel(II) Chloride
- Boron Sub-2,3-naphthalocyanine Chloride
- Boron Subphthalocyanine Chloride
- Boron Trichloride Dimethyloctylamine Complex
- Boron Trichloride Methyl Sulfide Complex
- Boron Trichloride Methyl Sulfide Complex Solution
- Butyltin Trichloride
- Cu(dap)2 Chloride
- Cyclopentadienylmolybdenum(IV) Tetrachloride
- Dibutyltin Dichloride
- Dichloro(norbornadiene)palladium(II)
- Dichlorobis(pyridine)palladium(II)
- Diethylaluminum Chloride
- Diethylgermanium Dichloride
- Di-i-butylaluminum Chloride
- Dimethylaluminum Chloride
- Dimethylgermanium Dichloride
- Dimethyltin Dichloride
- Di-n-butylgermanium Dichloride
- Diphenylgermanium Dichloride
- Diphenyltin Dichloride
- Di-tert-butyltin Dichloride
- Ethylaluminum Dichloride
- Ethylaluminum Sesquichloride
- Ethylgermanium Trichloride
- Ethylmagnesium Chloride
- Ethylmercuric Chloride
- Gallium(III) 2,3-naphthalocyanine Chloride
- Gallium(III) Phthalocyanine Chloride
- Gallium(III) Protoporphyrin IX Chloride
- Germanium(II) Chloride Dioxane Complex
- Hexylmagnesium Chloride
- Indium(III) Phthalocyanine Chloride
- Isopropylmagnesium Chloride
- Isopropylmagnesium Chloride Lithium Chloride Complex Solution
- Lithium Chloride Monohydrate
- Methallylnickel Chloride Dimer
- Methyl Green, Zinc Chloride Salt
- Methylammonium Chloride
- Methylgermanium Trichloride
- Methylmagnesium Chloride
- Methyltin Trichloride
- N-butylmagnesium Chloride
- Nickel(II) Chloride Ethylene Glycol Dimethyl Ether Complex
- Octylmagnesium Chloride
- o-Tolylmagnesium Chloride Solution
- Palladium(π-cinnamyl) Chloride Dimer
- Phenylgermanium Trichloride
- Phenylmagnesium Chloride
- Phenyltin Trichloride
- Propylmagnesium Chloride Solution
- Protoporphyrin IX Cobalt Chloride
- S-Acetamidomethyl-L-cysteine Hydrochloride
- sec-Butylmagnesium Chloride Lithium Chloride Complex Solution
- sec-Butylmagnesium Chloride Solution
- Silicon 2,3-naphthalocyanine Dichloride
- Silicon Phthalocyanine Dichloride
- Tert-butyl Magnesium Chloride
- Tetramethylammonium Chloride
- Tetraphenylarsonium(V) Chloride Hydrate
- Tetraphenylarsonium(V) Chloride Hydrochloride Hydrate
- Tin Protoporphyrin IX Dichloride
- Tin(IV) 2,3-naphthalocyanine Dichloride
- Tin(IV) bis(acetylacetonate) Dichloride
- Tin(IV) Phthalocyanine Dichloride
- Tributylgermanium Chloride
- Tributylmethylammonium Chloride
- Tributyltin Chloride
- Tricaprylylmethylammonium Chloride
- Tricyclohexyltin Chloride
- Triethylgermanium Chloride
- Triethyloxonium Hexachloroantimonate
- Trimethylgermanium Chloride
- Trimethyltin Chloride
- Trimethyltin Chloride Solution
- Triphenylantimony Dichloride
- Triphenylbismuth Dichloride
- Triphenylcarbenium Hexachloroantimonate
- Triphenylgermanium Chloride
- Triphenyltin Chloride
- Tris(2,2'-bipyrimide)ruthenium(II) Dichloride
- Tris(4-trifluoromethylphenyl)bismuth Dichloride
- Tris(ethylenediamine)cobalt(III) Chloride Trihydrate
- Vinylmagnesium Chloride Solution
- Zinc Chloride Tetrahydrofuran Complex
- Calcium-44 Chloride Isotope
- Iron-57 Chloride
- Cadmium-111 Chloride Isotope
- Cadmium-113 Chloride Isotope
- Calcium-44 Chloride Isotope
- Iron-57 Chloride
- Lithium-6 Chloride
- Potassium-40 Chloride Isotope
- Potassium-41 Chloride Isotope
- Rubidium-85 Chloride Isotope
- Rubidium-87 Chloride Isotope
- Sodium Chloride-35sup>Cl Isotope
Chlorine Properties
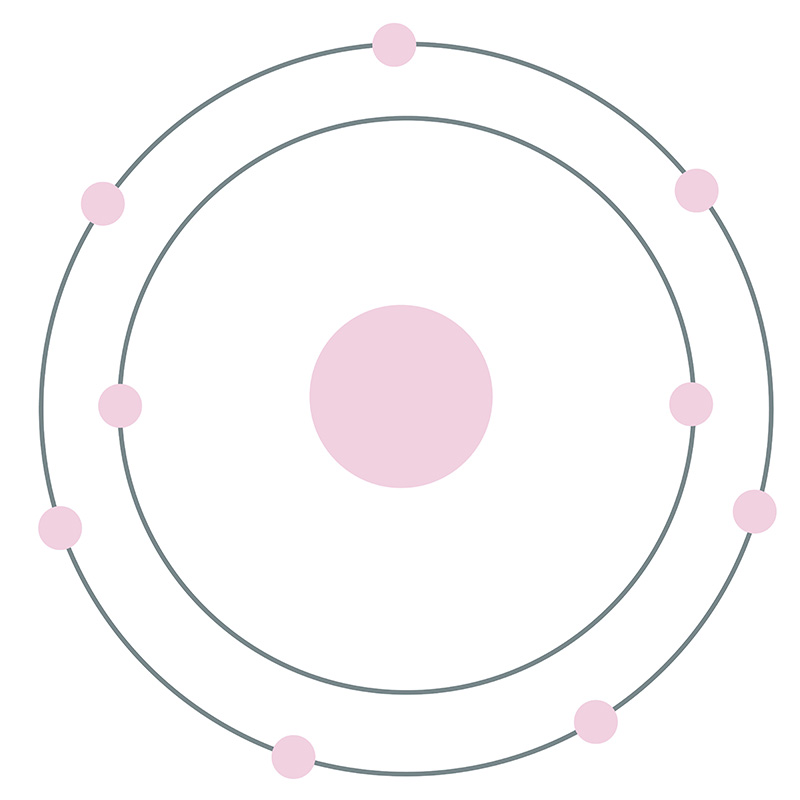
![]() Chlorine is a Block P, Group 17, Period 3 element. Its electron configuration is [Ne]3s23p5. The chlorine atom has a covalent radius of 102±4 pm and its Van der Waals radius is 175 pm. In its elemental form, CAS 7440-44-0, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine.
Chlorine is a Block P, Group 17, Period 3 element. Its electron configuration is [Ne]3s23p5. The chlorine atom has a covalent radius of 102±4 pm and its Van der Waals radius is 175 pm. In its elemental form, CAS 7440-44-0, chlorine is a yellow-green gas. Chlorine is the second lightest halogen after fluorine. 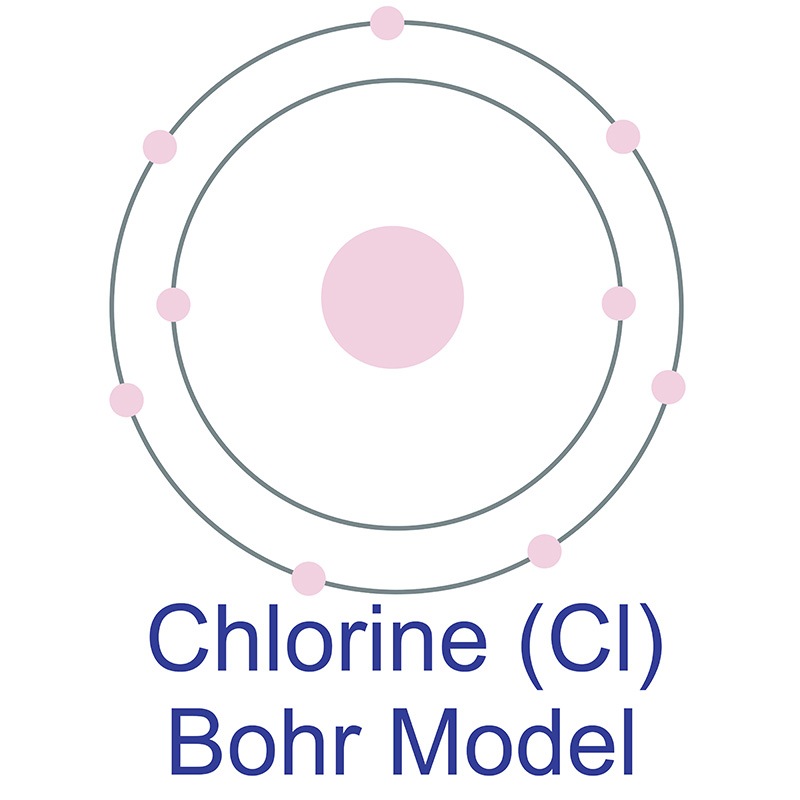 it has the third highest electronegativity and the highest electron affinity of all the elements making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808.
it has the third highest electronegativity and the highest electron affinity of all the elements making it a strong oxidizing agent. It is rarely found by itself in nature. Chlorine was discovered and first isolated by Carl Wilhelm Scheele in 1774. It was first recognized as an element by Humphry Davy in 1808.
Health, Safety & Transportation Information for Chlorine
Safety data for Chlorine and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental Chlorine.
| Safety Data | |
|---|---|
| Material Safety Data Sheet | MSDS |
| Signal Word | Danger |
| Hazard Statements | H270-H280-H315-H319-H331-H335-H400 |
| Hazard Codes | T,N |
| Risk Codes | 23-36/37/38-50 |
| Safety Precautions | 9-45-61 |
| RTECS Number | FO2100000 |
| Transport Information | UN 1017 2.3 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Chlorine Isotopes
Chlorine has two stable isotopes: 35Cl and 37Cl.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 28Cl | 28.02851(54)# | N/A | p to 27S | (1+)# | N/A | 181.04 | - |
| 29Cl | 29.01411(21)# | <20 ns | p to 28S | (3/2+)# | N/A | 202.16 | - |
| 30Cl | 30.00477(21)# | <30 ns | p to 29S | (3+)# | N/A | 219.56 | - |
| 31Cl | 30.99241(5) | 150(25) ms | ß+ to 31S; ß+ + p to 30P | 3/2+ | N/A | 238.82 | - |
| 32Cl | 31.985690(7) | 298(1) ms | ß+ to 32S; ß+ + a to 28Si; ß+ + p to 31P | 1+ | N/A | 253.42 | - |
| 33Cl | 32.9774519(5) | 2.511(3) s | ß+ to 33S | 3/2+ | N/A | 268.95 | - |
| 34Cl | 33.97376282(19) | 1.5264(14) s | ß+ to 34S | 0+ | N/A | 280.76 | - |
| 35Cl | 34.96885268(4) | STABLE | - | 3/2+ | 0.8218736 | 293.49 | 75.78 |
| 36Cl | 35.96830698(8) | 3.01(2)E+5 y | ß- to 36Ar | 2+ | 1.28547 | 301.57 | - |
| 37Cl | 36.96590259(5) | STABLE | - | 3/2+ | 0.684123 | 312.44 | 24.22 |
| 38Cl | 37.96801043(10) | 37.24(5) min | ß- to 38Ar | 2- | 2.05 | 317.73 | - |
| 39Cl | 38.9680082(19) | 55.6(2) min | ß- to 39Ar | 3/2+ | N/A | 325.81 | - |
| 40Cl | 39.97042(3) | 1.35(2) min | ß- to 40Ar | 2- | N/A | 332.02 | - |
| 41Cl | 40.97068(7) | 38.4(8) s | ß- to 41Ar | (1/2+,3/2+) | N/A | 340.1 | - |
| 42Cl | 41.97325(15) | 6.8(3) s | ß- to 42Ar | N/A | N/A | 345.38 | - |
| 43Cl | 42.97405(17) | 3.07(7) s | ß- to 43Ar; ß- + n to 42Ar | 3/2+# | N/A | 352.53 | - |
| 44Cl | 43.97828(12) | 0.56(11) s | ß- to 44Ar; ß- + n to 43Ar | N/A | N/A | 356.88 | - |
| 45Cl | 44.98029(13) | 400(40) ms | ß- to 45Ar; ß- + n to 44Ar | 3/2+# | N/A | 363.1 | - |
| 46Cl | 45.98421(77) | 232(2) ms | ß- + n to 45Ar; ß- to 46Ar | N/A | N/A | 367.45 | - |
| 47Cl | 46.98871(64)# | 101(6) ms | ß- to 47Ar; ß- + n to 46Ar | 3/2+# | N/A | 371.8 | - |
| 48Cl | 47.99495(75)# | 100# ms [>200 ns] | ß- to 48Ar | N/A | N/A | 374.29 | - |
| 49Cl | 49.00032(86)# | 50# ms [>200 ns] | ß- to 49Ar | 3/2+# | N/A | 376.78 | - |
| 50Cl | 50.00784(97)# | 20# ms | ß- to 50Ar | N/A | N/A | 378.34 | - |
| 51Cl | 51.01449(107)# | 2# ms [>200 ns] | ß- to 51Ar | 3/2+# | N/A | 379.9 | - |