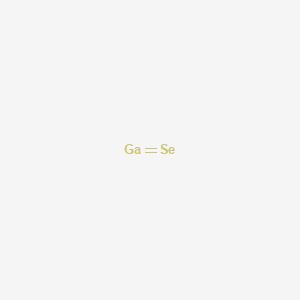SECTION 1. IDENTIFICATION
Product Name: Gallium Selenide Single Crystal
Product Number: All applicable American Elements product codes, e.g. GA-SE-04-ISX
, GA-SE-045-ISX
, GA-SE-05-ISX
, GA-SE-06-ISX
CAS #: 12024-11-2
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008:
GHS06: Skull and crossbones
GHS08: Health hazard
Acute Tox. 2 H300 Fatal if swallowed.
Acute Tox. 2 H330 Fatal if inhaled.
STOT RE 2 H373 May cause damage to the central nervous system, the liver and the digestive system
through prolonged or repeated exposure. Route of exposure: Oral, Inhalative.
Classification according to Directive 67/548/EEC or Directive 1999/45/EC:
T; Toxic
N; Dangerous for the environment
R23/25: Toxic by inhalation and if swallowed.
R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic
environment.
R33: Danger of cumulative effects.
Information concerning particular hazards for human and environment: N/A
Hazards not otherwise classified: No data available
Label elements
Labeling according to Regulation (EC) No 1272/2008
The substance is classified and labeled according to the CLP regulation.
Hazard:


GHS06, GHS08
Signal word - Danger
Hazard statements:
H300+H330 Fatal if swallowed or if inhaled.
H373 May cause damage to the central nervous system, the liver and the digestive system
through prolonged or repeated exposure. Route of exposure: Oral, Inhalative.
Precautionary statements:
P260 Do not breathe dust/fume/gas/mist/vapors/spray.
P284 [In case of inadequate ventilation] wear respiratory protection.
P301+P310 IF SWALLOWED: Immediately call a POISON CENTER/ doctor/...
P320 Specific treatment is urgent (see on this label).
P405 Store locked up.
P501 Dispose of contents/container in accordance with local/regional/national/international
regulations.
WHMIS classification:
D1A Very toxic material causing immediate and serious toxic effects
Classification system
HMIS ratings (scale 0-4)
(Hazardous Materials Identification System):
HEALTH 2 Health (acute effects) = 2
FIRE 0 Flammability = 0
REACTIVITY 1 Physical Hazard = 1
Other hazards
Results of PBT and vPvB assessment:
PBT: N/A
vPvB: N/A
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Description: Gallium Monoselenide
Synonyms: Gallium(II) selenide; hydrido(selenoxo)gallium
Formula: GaSe
Molecular weight: 148.68
CAS number: 12024-11-2
SECTION 4. FIRST AID MEASURES
Description of first aid measures
General information:
Immediately remove any clothing soiled by the product. Remove breathing apparatus only after contaminated
clothing has been completely removed. In case of irregular breathing or respiratory arrest provide artificial
respiration.
If inhaled:
Supply patient with fresh air. If not breathing, provide artificial respiration. Keep patient warm. Seek immediate medical advice.
In case of skin contact:
Immediately wash with soap and water; rinse thoroughly. Seek immediate medical advice.
In case of eye contact:
Rinse opened eye for several minutes under running water. Consult a physician.
If swallowed:
Do not induce vomiting; immediately call for medical help.
Most important symptoms and effects, both acute and delayed:
No data available
Indication of any immediate medical attention and special treatment needed:
No data available
SECTION 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing agents:
Product is not flammable. Use fire-fighting measures that suit the surrounding fire.
Special hazards arising from the substance or mixture:
If this product is involved in a fire, the following can be released: Selenium dioxide (SeO2), Hydrogen selenide
Advice for firefighters
Protective equipment:
Wear self-contained respirator. Wear fully protective impervious suit.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Keep unprotected persons away. Ensure adequate ventilation
Environmental precautions:
Do not allow material to be released to the environment without official permits. Do not allow
product to reach sewage system or any water course. Do not allow material to penetrate the ground or soil.
Methods and materials for containment and cleanup:
Dispose contaminated material as waste according to item 13. Ensure adequate ventilation.
Prevention of secondary hazards:
No special measures required.
Reference to other sections:
See Section 7 for information on safe handling.
See Section 8 for information on personal protection equipment.
See Section 13 for disposal information.
SECTION 7. HANDLING AND STORAGE
Handling
Precautions for safe handling:
Keep container tightly sealed. Store in cool, dry place in tightly closed containers. Ensure good ventilation at the
workplace. Open and handle container with care.
Information about protection against explosions and fires:
The product is not flammable
Conditions for safe storage, including any incompatibilities
Requirements to be met by storerooms and receptacles:
No special requirements.
Information about storage in one common storage facility:
Store away from oxidizing agents.
Further information about storage conditions:
Keep container tightly sealed. Store in cool, dry conditions in well-sealed containers. Store at room temperature.
Specific end use(s):
No data available
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Additional information about design of technical systems:
Properly operating chemical fume hood designed for hazardous chemicals and having an average face velocity of
at least 100 feet per minute.
Control parameters
Components with limit values that require monitoring at the workplace:
Selenium and selenium compounds (as Se)
mg/m3
ACGIH TLV 0.2
Austria MAK 0.1
Belgium TWA 0.2
Denmark TWA 0.1
Finland TWA 0.1; 0.3-STEL
Germany MAK 0.1
Hungary 0.1-STEL
Japan OEL 0.1
Korea TLV 0.2
Netherlands MAC-TGG 0.2
Poland TWA 0.1; 0.3-STEL
Sweden NGV 0.1
Switzerland MAK-W 0.1
United Kingdom TWA 0.1
USA PEL 0.2
Additional information: No data
Exposure controls
Personal protective equipment
General protective and hygienic measures:
The usual precautionary measures for handling chemicals should be followed. Keep away from foodstuffs,
beverages and feed. Remove all soiled and contaminated clothing immediately. Wash hands before breaks and at
the end of work. Store protective clothing separately. Maintain an ergonomically appropriate working environment.
Breathing equipment:
Use self-contained respiratory protective device in emergency situations.
Protection of hands:
Impervious gloves. Inspect gloves prior to use. The selection of suitable
gloves not only depends on the material, but also on quality. Quality will vary from manufacturer to manufacturer.
Eye protection:
Safety glasses
Body protection:
Protective work clothing.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
Form: Solid
Odor: No data available
Odor threshold: No data available
pH: N/A
Melting point range: No data available
Boiling point range: No data available
Sublimation temperature/start: No data available
Flash point: N/A
Flammability: No data available
Ignition temperature: No data available
Decomposition temperature: No data available
Autoignition: No data available
Danger of explosion: No
Lower explosion limits: No data available
Upper explosion limits: No data available
Vapor pressure: N/A
Density: No data available
Relative density: No data available
Vapor density: N/A
Evaporation rate: N/A
Solubility in water: No data available
Dynamic viscosity: N/A
Partition coefficient (n-octanol/water): No data available
Kinematic viscosity: N/A
SECTION 10. STABILITY AND REACTIVITY
Reactivity
No data available
Chemical stability:
Stable under recommended storage conditions.
Thermal decomposition / conditions to be avoided:
Decomposition will not occur if used and stored according to specifications.
Possibility of hazardous reactions:
No dangerous reactions known
Incompatible materials:
Oxidizing agents
Hazardous decomposition products:
Selenium dioxide (SeO2), Hydrogen selenide
SECTION 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity:
Fatal if inhaled. Fatal if swallowed.
LD/LC50 values that are relevant for classification:
No data
Skin irritation or corrosion:
May cause irritation
Eye irritation or corrosion:
May cause irritation
Sensitization:
No sensitizing effects known.
Germ cell mutagenicity:
No effects known.
Carcinogenicity:
No classification data on carcinogenic properties of this material is available from the EPA, IARC, NTP, OSHA or ACGIH.
Reproductive toxicity:
No effects known.
Specific target organ system toxicity - repeated exposure:
May cause damage to the central nervous system, the liver and the digestive system through prolonged or
repeated exposure. Route of exposure: Oral, Inhalative.
Specific target organ system toxicity - single exposure:
No effects known.
Aspiration hazard:
No effects known.
Subacute to chronic toxicity:
Selenium may cause amyotrophic lateral sclerosis, bronchial irritation, gastrointestinal distress, vasopharyngeal
irritation, garlic odor on breath and sweat, metallic taste, pallor, irritability, excessive fatigue, loss of fingernails
and hair, pulmonary edema, anemia and weight loss.
Additional toxicological information:
To the best of our knowledge the acute and chronic toxicity of this substance is not fully known.
SECTION 12. ECOLOGICAL INFORMATION
Toxicity
Aquatic toxicity:
No data available
Persistence and degradability:
No data available
Behavior in environmental systems:
Bioaccumulative potential:
No data available
Mobility in soil:
No data available
Ecotoxical effects:
Remark: Very toxic for aquatic organisms
Additional ecological information:
Do not allow material to be released to the environment without official permits. Do not allow
product to reach groundwater, water courses, or sewage systems, even in small quantities. Danger to drinking
water if even extremely small quantities leak into the ground. Also poisonous for fish and plankton in water
bodies. May cause long lasting harmful effects to aquatic life. Avoid transfer into the environment. Very toxic for
aquatic organisms.
Results of PBT and vPvB assessment:
PBT: N/A
vPvB: N/A
Other adverse effects:
No data available
SECTION 13. DISPOSAL CONSIDERATIONS
Waste treatment methods:
Recommendation Consult official regulations to ensure proper disposal.
Uncleaned packagings:
Recommendation: Disposal must be made according to official regulations.
SECTION 14. TRANSPORT INFORMATION
UN-Number
DOT, ADR, IMDG, IATA UN3283
UN proper shipping name
DOT Selenium Compound, N.O.S. (Gallium Monoselenide), 6.1, PG III
ADR 3283 Selenium Compound, N.O.S. (Gallium Monoselenide), 6.1, PG III
IMDG, IATA SELENIUM COMPOUND, N.O.S. (Gallium Monoselenide)
Transport hazard class(es)
DOT, IMDG
Class 6.1
Label 6.1
ADR
Class 6.1
Label 6.1
IATA
Class 6.1
Label 6.1
Packing group
DOT, ADR, IMDG, IATA III
Environmental hazards:
Special marking (ADR): Symbol (fish and tree)
Special marking (IATA): Symbol (fish and tree)
Special precautions for user Warning: Miscellaneous dangerous substances and articles
Danger code (Kemler): 90
EMS Number: F-A,S-F
Transport in bulk according to Annex II of MARPOL73/78 and the IBC Code: N/A
Transport/Additional information:
DOT
Marine Pollutant (DOT): No
UN "Model Regulation": UN3283, Selenium Compound, N.O.S. (Gallium Monoselenide), 6.1, PG III
SECTION 15. REGULATORY INFORMATION
No data available
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 Gallium was predicted by Dmitri Mendeleev in 1871. It was first discovered and isolated by Lecoq de Boisbaudran in 1875. In its elemental form, gallium has a silvery appearance.
Gallium was predicted by Dmitri Mendeleev in 1871. It was first discovered and isolated by Lecoq de Boisbaudran in 1875. In its elemental form, gallium has a silvery appearance.  Gallium is one of three elements that occur naturally as a liquid at room temperature, the other two being
Gallium is one of three elements that occur naturally as a liquid at room temperature, the other two being  See more Selenium products.
See more Selenium products. One of the most common uses for selenium is in
One of the most common uses for selenium is in