About Cesium
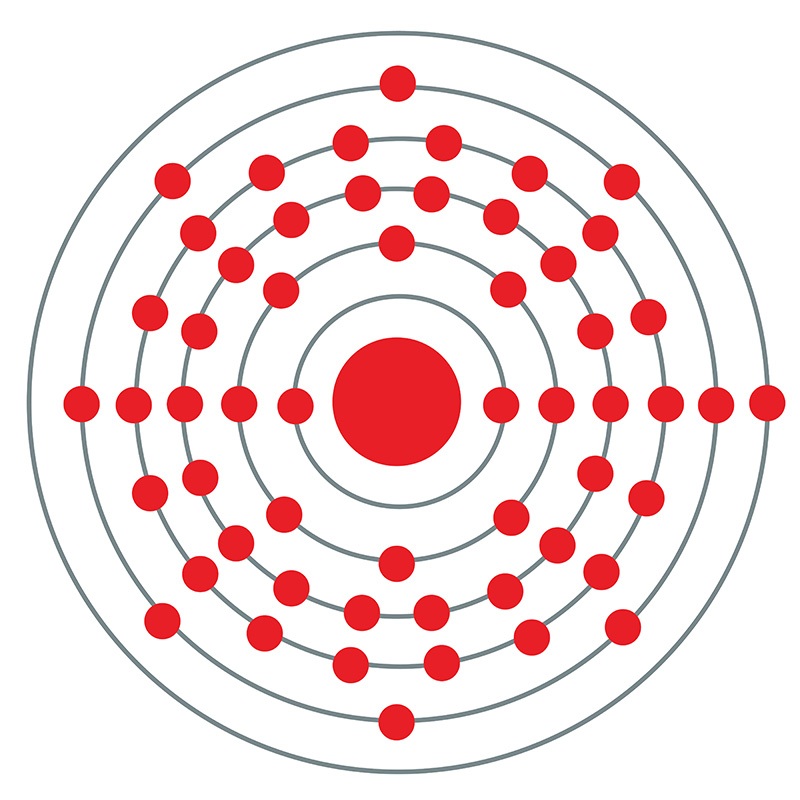
With one electron in its sixth and outermost shell, cesium (or caesium) is the most electropositive of all stable elements on the periodic table: the metal is extremely pyrophoric, spontaneously igniting when it comes into contact with air and exploding violently in water or ice at any temperature above -116 °C. Though cesium is only mildly toxic, it is classified as hazardous because of its high reactivity and is usually packaged in glass ampoules in a vacuum or under an inert gas such as argon. The heaviest of the stable alkali metals, cesium is silvery-gold in appearance, ductile, and the softest element on the periodic table at 0.2 on the Mohs scale. Cesium melts at 28 °C, making it one of three elements (the others being gallium and mercury) that are liquid at or near room temperature; mercury is the only element with a lower melting point. Only one of cesium’s known isotopes is stable (133Cs), but its total of 39 makes it tied with xenon as the two elements with the most amount of known isotopes. Cesium was also the first element to be discovered via spectroscopy by German scientists Robert Bunsen and Gustav Kirchhoff, who had invented the technology the previous year. Bunsen and Kirchhoff gave the new element a name based on the Latin word caesius (sky or heavenly blue) for the two brilliant blue lines emitted in its spectrum.
Cesium is the 45th most abundant element on earth, found in the minerals pollucite, avogadrite, pezzottaite, londonite, rhodizite, beryl, and some potassium ores; the primary commercial source of metallic cesium is from the mining of pollucite, while cesium radioisotopes are produced from nuclear reactor waste. Oxygen-free metallic cesium can also be produced via the thermal decomposition of cesium azide (CsN3). Laboratories use cesium compounds for various organic chemistry functions such as the hydrogenation of organic compounds or, in the case of cesium fluoride, as a source of the fluorine anion. X-ray radiotherapy in cancer treatment often employs radioactive isotope cesium-137. In commercial and industrial applications, cesium salts can serve as catalyst promoters, glass strengtheners, components of photoelectric cells, crystals in scintillation counters, and “getters” in vacuum tubes; cesium formate brines are commonly utilized in oil drilling to lubricate drill bits and maintain pressure. Thermionic energy converters use a vapor of cesium ions to determine the work function of the electrodes. Early ion propulsion engines for space exploration used cesium as a propellant until xenon became the standard. The most accurate commercially available atomic clocks keep time using the oscillation of the cesium-133 atom’s 9193 MHz hyperfine transition frequency. Known as the “cesium standard,” this frequency is the primary time standard for the definition of the second and is critical to the data transmission infrastructures of cell phone networks, GPS, and the internet.
Products
Cesium is used as an oxygen "getter" in vacuum and electronic tubes and as a component of photoelectric cells. Atomic clocks use the microwave spectral line emitted by Cesium-133 for reference. Radioactive Cesium-137 is an artificial isotope that is used for cancer radiotherapy.  Laboratories use various cesium compounds for their ability to hydrogenate organic compounds. Cesium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cesium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Laboratories use various cesium compounds for their ability to hydrogenate organic compounds. Cesium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Nanoparticles and nanopowders provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Oxides are available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cesium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Cesium Properties
![]() Cesium (or Caesium ) is a Block S, Group 1, Period 6 element. The number of electrons in each of Cesium's shells is 2, 8, 18, 18, 8, 1 and its electronic configuration is [Xe] 6s1. The cesium atom has a radius of 265.5.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-46-2, cesium has a silvery gold appearance. Cesium is a member of the alkali group of metals. It is one of three metals that occur as a liquid at room
Cesium (or Caesium ) is a Block S, Group 1, Period 6 element. The number of electrons in each of Cesium's shells is 2, 8, 18, 18, 8, 1 and its electronic configuration is [Xe] 6s1. The cesium atom has a radius of 265.5.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7440-46-2, cesium has a silvery gold appearance. Cesium is a member of the alkali group of metals. It is one of three metals that occur as a liquid at room 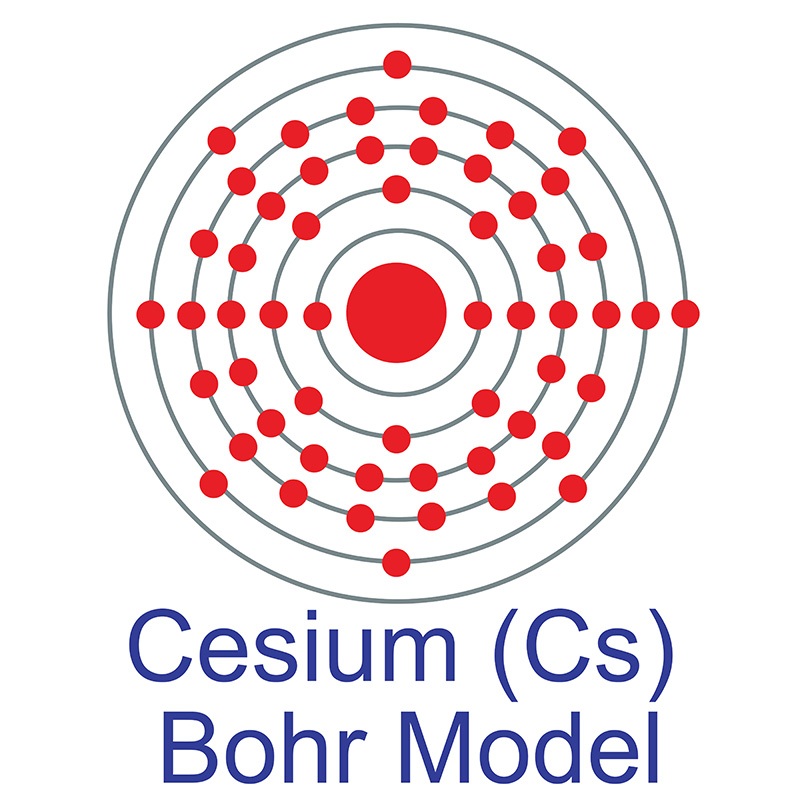 high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
Health, Safety & Transportation Information for Cesium
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H260-H314 |
| Hazard Codes | F,C |
| Risk Codes | 11-14/15-34 |
| Safety Precautions | 8-16-26-36/37/39-43-45 |
| RTECS Number | FK9225000 |
| Transport Information | UN 1415 4.3/PG 1 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Cesium Isotopes
Cesium (Caesium, Cs) has 40 known isotopes with atomic masses ranging from 112 to 151. 133Cs is the only stable isotope.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 112Cs | 111.95030(33)# | 500(100) µs | p to 111Xe; a to 108I | 1+# | N/A | 889.68 | - |
| 113Cs | 112.94449(11) | 16.7(7) µs | p to 112Xe; ß+ to 113Xe | 5/2+# | N/A | 907.08 | - |
| 114Cs | 113.94145(33)# | 0.57(2) s | ß+ to 114Xe; ß+ + p to 113I; ß+ + a to 110Te; a to 110I | (1+) | N/A | 915.16 | - |
| 115Cs | 114.93591(32)# | 1.4(8) s | ß+ to 115Xe; ß+ + p to 114I | 9/2+# | N/A | 932.55 | - |
| 116Cs | 115.93337(11)# | 0.70(4) s | ß+ to 116Xe; ß+ + p to 115I; ß+ + a to 112Te | (1+) | N/A | 940.63 | - |
| 117Cs | 116.92867(7) | 8.4(6) s | ß+ to 117Xe | (9/2+)# | N/A | 958.03 | - |
| 118Cs | 117.926559(14) | 14(2) s | ß+ to 118Xe; ß+ + p to 117I; ß+ + a to 114Te | 2 | N/A | 966.1 | - |
| 119Cs | 118.922377(15) | 43.0(2) s | ß+ to 119Xe; ß+ + a to 115Te | 9/2+ | N/A | 974.18 | - |
| 120Cs | 119.920677(11) | 61.2(18) s | ß+ to 120Xe; ß+ + a to 116Te; ß+ + p to 118I | 2(-#) | N/A | 982.26 | - |
| 121Cs | 120.917229(15) | 155(4) s | ß+ to 121Xe | 3/2(+) | N/A | 999.66 | - |
| 122Cs | 121.91611(3) | 21.18(19) s | ß+ to 122Xe; ß+ + a to 118Te | 1+ | N/A | 1007.74 | - |
| 123Cs | 122.912996(13) | 5.88(3) min | ß+ to 123Xe | 1/2+ | N/A | 1015.81 | - |
| 124Cs | 123.912258(9) | 30.9(4) s | ß+ to 124Xe | 1+ | N/A | 1023.89 | - |
| 125Cs | 124.909728(8) | 46.7(1) min | ß+ to 125Xe | 1/2(+) | N/A | 1041.29 | - |
| 126Cs | 125.909452(13) | 1.64(2) min | ß+ to 126Xe | 1+ | N/A | 1049.37 | - |
| 127Cs | 126.907418(6) | 6.25(10) h | ß+ to 127Xe | 1/2+ | N/A | 1057.45 | - |
| 128Cs | 127.907749(6) | 3.640(14) min | ß+ to 128Xe | 1+ | N/A | 1065.52 | - |
| 129Cs | 128.906064(5) | 32.06(6) h | EC to 129Xe | 1/2+ | 1.49 | 1073.6 | - |
| 130Cs | 129.906709(9) | 29.21(4) min | EC to 130Xe; ß- to 130Ba | 1+ | 1.46 | 1081.68 | - |
| 131Cs | 130.905464(5) | 9.689(16) d | EC to 131Xe | 5/2+ | 3.54 | 1089.76 | - |
| 132Cs | 131.9064343(20) | 6.480(6) d | EC to 132Xe; ß- to 132Ba | 2+ | 2.22 | 1097.84 | - |
| 133Cs | 132.905451933(24) | STABLE | - | 7/2+ | 2.582024 | 1105.92 | 100 |
| 134Cs | 133.906718475(28) | 2.0652(4) y | EC to 134Xe; ß- to 134Ba | 4+ | 2.994 | 1114 | - |
| 135Cs | 134.9059770(11) | 2.3(3)E+6 y | ß- to 135Ba | 7/2+ | 2.732 | 1122.08 | - |
| 136Cs | 135.9073116(20) | 13.16(3) d | ß- to 136Ba | 5+ | 3.71 | 1130.15 | - |
| 137Cs | 136.9070895(5) | 30.1671(13) y | ß- to 137Ba | 7/2+ | 2.84 | 1138.23 | - |
| 138Cs | 137.911017(10) | 33.41(18) min | ß- to 138Ba | 3- | N/A | 1136.99 | - |
| 139Cs | 138.913364(3) | 9.27(5) min | ß- to 139Ba | 7/2+ | N/A | 1145.07 | - |
| 140Cs | 139.917282(9) | 63.7(3) s | ß- to 140Ba | 1- | N/A | 1153.15 | - |
| 141Cs | 140.920046(11) | 24.84(16) s | ß- to 141Ba; ß- + n to 140Ba | 7/2+ | N/A | 1151.91 | - |
| 142Cs | 141.924299(11) | 1.689(11) s | ß- to 142Ba; ß- + n to 141Ba | 0- | N/A | 1159.99 | - |
| 143Cs | 142.927352(25) | 1.791(7) s | ß- to 143Ba; ß- + n to 142Ba | 3/2+ | N/A | 1168.07 | - |
| 144Cs | 143.932077(28) | 994(4) ms | ß- to 144Ba; ß- + n to 143Ba | 1(-#) | N/A | 1166.83 | - |
| 145Cs | 144.935526(12) | 582(6) ms | ß- to 145Ba; ß- + n to 144Ba | 3/2+ | N/A | 1174.91 | - |
| 146Cs | 145.94029(8) | 0.321(2) s | ß- to 146Ba; ß- + n to 145Ba | 1- | N/A | 1173.68 | - |
| 147Cs | 146.94416(6) | 0.235(3) s | ß- to 146Ba; ß- + n to 147Ba | (3/2+) | N/A | 1181.75 | - |
| 148Cs | 147.94922(62) | 146(6) ms | ß- to 147Ba; ß- + n to 146Ba | N/A | N/A | 1189.83 | - |
| 149Cs | 148.95293(21)# | 150# ms [>50 ms] | ß- to 148Ba; ß- + n to 147Ba | 3/2+# | N/A | 1188.6 | - |
| 150Cs | 149.95817(32)# | 100# ms [>50 ms] | ß- to 149Ba; ß- + n to 148Ba | N/A | N/A | 1196.67 | - |
| 151Cs | 150.96219(54)# | 60# ms [>50 ms] | ß- to 150Ba; ß- + n to 149Ba | 3/2+# | N/A | 1195.44 | - |