SECTION 1. IDENTIFICATION
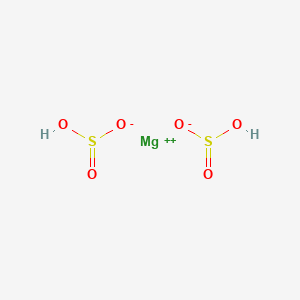
Product Name: Magnesium Bisulfite Solution
Product Number: All applicable American Elements product codes, e.g. MG-SIT2-02-SOL
, MG-SIT2-03-SOL
, MG-SIT2-04-SOL
, MG-SIT2-05-SOL
CAS #: 13774-25-9
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
2.1 Classification of the substance or mixture
GHS Classification in accordance with 29 CFR 1910 (OSHA HCS)
Corrosive to metals (Category 1), H290
Skin corrosion (Category 1B), H314
Serious eye damage (Category 1), H318
For the full text of the H-Statements mentioned in this Section, see Section 16.
2.2 GHS Label elements, including precautionary statements
Pictogram

Signal word Danger
Hazard statement(s)
H290 May be corrosive to metals.
H314 Causes severe skin burns and eye damage.
H318 Causes serious eye damage.
Precautionary statement(s)
P234 Keep only in original container.
P264 Wash skin thoroughly after handling.
P280 Wear protective gloves/ protective clothing/ eye protection/ face protection.
P301 + P330 + P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P303 + P361 + P353 IF ON SKIN (or hair): Remove/ Take off immediately all contaminated clothing. Rinse skin with water/ shower.
P304 + P340 + P310 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. Immediately call a POISON CENTER or doctor/ physician.
P305 + P351 + P338 + P310 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER or doctor/ physician.
P363 Wash contaminated clothing before reuse.
P390 Absorb spillage to prevent material damage.
P405 Store locked up.
P406 Store in corrosive resistant stainless steel container with a resistant inner liner.
P501 Dispose of contents/ container to an approved waste disposal plant.
2.3 Hazards not otherwise classified (HNOC) or not covered by GHS
Contact with acids liberates toxic gas.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
,Component
Magnesium Bisulfite
CAS Number
13774-25-9
% by Wt.
30 %,
SECTION 4. FIRST AID MEASURES
,Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes while holding eyelids open. Tilt head to avoid contaminating unaffected eye. Get immediate medical attention.
Skin Contact: Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical attention immediately. Do not reuse clothing and shoes until cleaned.
Inhalation: Remove to fresh air. If breathing is difficult, administer oxygen. If not breathing, give artificial respiration, preferably mouth-to-mouth. GET MEDICAL ATTENTION IMMEDIATELY.
Ingestion: If fully conscious, drink a quart of water. DO NOT induce vomiting. CALL A PHYSICIAN
IMMEDIATELY. If unconscious or in convulsions, take immediately to a hospital or a physician. NEVER induce vomiting or give anything by mouth to an unconscious victim. If vomiting occurs spontaneously, keep head below hips to prevent aspiration of liquid into the lungs.
Most Important Symptoms/Effects:
Eye Contact: CORROSIVE-Causes severe irritation and burns. May cause: tissue destruction. permanent eye damage. blindness.
Skin Contact: CORROSIVE-Causes severe irritation and burns. Contact may cause: tissue destruction.
Skin Absorption: May be harmful if absorbed through skin.
Inhalation: CORROSIVE-Causes severe irritation and burns. May irritate: mucous membranes. respiratory tract.
May cause: burning sensation. coughing. wheezing. laryngitis. shortness of breath. headache. nausea. vomiting. spasm. inflammation and edema of the larynx and bronchi. chemical pneumonitis. pulmonary edema. May cause damage to the: upper respiratory tract. lungs.
Ingestion: CORROSIVE-Causes severe irritation and burns. Ingestion can cause very serious damage to the mouth, esophogus, stomach, and other tissues with which contact is made, and may be fatal. May cause: headache. nausea. vomiting. May cause severe burns to the: digestive tract. Very large doses cause violent colic, diarrhea, depression and death.,
SECTION 5. FIREFIGHTING MEASURES
,Extinguishing Media: For fires in area use appropriate media. For example: Dry chemical. Carbon dioxide. Foam.
Fire Fighting Methods: Evacuate area of unprotected personnel. Wear protective clothing including NIOSH approved self-contained breathing apparatus. Remain upwind of fire to avoid hazardous vapors and decomposition products. Use water spray to cool fire-exposed containers. Run-off from fire control may cause pollution.
Fire and Explosion Hazards: None known. Emits toxic fumes under fire conditions.
Hazardous Combustion Products: Toxic vapors. Sulfur oxides. Sulfur Dioxide gas will be released at a rate increasing with temperature.,
SECTION 6. ACCIDENTAL RELEASE MEASURES
,Spill Clean-Up Procedures: CORROSIVE MATERIAL. Evacuate unprotected personnel from area. Maintain adequate ventilation. Follow personal protective equipment recommendations found in Section 8. Never exceed any occupational exposure limit. Contain spill, place into drums for proper disposal. Flush remaining area with water and neutralize with Soda Ash or Lime and dispose of properly. Sulfur dioxide and carbon dioxide may be released during neutralization. Avoid direct discharge to sewers and surface waters. Notify authorities if entry occurs.,
SECTION 7. HANDLING AND STORAGE
,Handling: Avoid contact with eyes, skin, and clothing. Use with adequate ventilation. Do not swallow. Avoid breathing vapors, mists, or dust. Do not eat, drink, or smoke in work area. Wash thoroughly after handling.
Empty containers retain product residue (vapor, dust, or liquid) and can be dangerous. DO NOT pressurize, cut, weld, braze, solder, drill, grind, or expose such containers to heat, flame, sparks, static electricity, or other source of ignition. They may explode and cause injury or death.
Storage: CORROSIVE MATERIAL. Store in a cool, well ventilated area, out of direct sunlight. Store in a dry location away from heat. Keep away from incompatible materials. Keep containers tightly closed. Do not store in unlabeled or mislabeled containers. Do not freeze. Relieve pressure in drums weekly. See Section 10 for incompatible materials.,
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
,OSHA Exposure Guidelines:
Component Limits
No components found.
ACGIH Exposure Guidelines:
Component Limits
No components found.
Note: Sulfur Dioxide gas may be released. The Exposure Limits for Sulfur Dioxide are: 5 ppm-TWA (OSHA); 2 ppm-TWA, 5 ppm-STEL (ACGIH)(Vacated 1989 OSHA PELs).
Engineering Controls: Local exhaust ventilation, process enclosures, or other engineering controls are imperative when handling or using this product to avoid overexposure. Avoid creating dust or mist. Maintain adequate ventilation. Do not use in closed or confined spaces. Keep levels below exposure limits. To determine exposure levels, monitoring should be performed regularly.
Eye/Face Protection: Wear chemical safety goggles and a full face shield while handling this product. Do not wear contact lenses.
Skin Protection: Prevent contact with this product. Wear gloves and protective clothing depending on condition of use. Protective gloves: Rubber (latex). Polyvinyl chloride.
Respiratory Protection: Respiratory protection must be worn when handling this product. If exposure limits are exceeded, wear: NIOSH-Approved respirator for dusts, mists, and/or SO2 vapors as conditions indicate. NIOSH Approved air-purifying respirator with: Acid gas cartridge. NIOSH-Approved self-contained breathing apparatus.
NIOSH-Approved positive pressure supplied air respirator. DO NOT exceed limits established by the respirator manufacturer. All respiratory protection programs must comply with OSHA 29 CFR 1910.134 and ANSI Z88.2 requirements and must be followed whenever workplace conditions require a respirator's use.
Other Protective Equipment: Eye-wash station. Safety shower. Rubber apron. Chemical safety shoes. Rubber boots. Protective clothing.
General Hygiene Conditions: Wash with soap and water before meal times and at the end of each work shift. Good manufacturing practices require gross amounts of any chemical be removed from skin as soon as practical, especially before eating or smoking.,
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
,Physical State: Liquid.
Color: Clear. Yellow.
Odor: Sulfur dioxide odor.
Odor Threshold: N.D.
pH: 2.50 (as is)
Freezing Point (deg. F): 5 (crystallization point)
Melting Point (deg. F): N.D.
Initial Boiling Point or Boiling Range: N.D.
Flash Point: NONE.
Flash Point Method: N.A.
Evaporation Rate (nBuAc = 1): N.D.
Flammability (solid, gas): N.D.
Lower Explosion Limit: N.A.
Upper Explosion Limit: N.A.
Vapor Pressure (mm Hg): ~9 @ 20C (SO2)
Vapor Density (air=1): N.D.
Specific Gravity or Relative Density: 1.25 @ 25C
Solubility in Water: Complete
Partition Coefficient (n-octanol/water): N.D.
Autoignition Temperature: No Data
Decomposition Temperature: N.D.
Viscosity: N.D.
% Volatile (wt%): N.D.
VOC (wt%): 0
VOC (lbs/gal): 0
Fire Point: N.D.,
SECTION 10. STABILITY AND REACTIVITY
,Reactivity: No data available.
Chemical Stability: Stable under normal conditions.
Possibility of Hazardous Reactions: Hazardous polymerization will not occur under normal conditions.
Conditions to Avoid: Avoid contact with heat, sparks, electric arcs, other hot surfaces, and open flames.
Incompatible Materials: Oxidizing agents. Acids. Alkalies.
Hazardous Decomposition Products: Toxic vapors. Sulfur oxides. Sulfur dioxide gas. Reacts with acids to release sulfur dioxide. Some sulfur dioxide gas evolves on standing.,
SECTION 11. TOXICOLOGICAL INFORMATION
,Component
No components found or no data available for product.
Routes of Exposure: Eyes. Skin. Inhalation. Ingestion.
Eye Contact: CORROSIVE-Causes severe irritation and burns. May cause: tissue destruction. permanent eye damage. blindness.
Skin Contact: CORROSIVE-Causes severe irritation and burns. Contact may cause: tissue destruction.
Skin Absorption: May be harmful if absorbed through skin.
Inhalation: CORROSIVE-Causes severe irritation and burns. May irritate: mucous membranes. respiratory tract. May cause: burning sensation. coughing. wheezing. laryngitis. shortness of breath. headache. nausea. vomiting. spasm. inflammation and edema of the larynx and bronchi. chemical pneumonitis. pulmonary edema. May cause damage to the: upper respiratory tract. lungs.
Ingestion: CORROSIVE-Causes severe irritation and burns. Ingestion can cause very serious damage to the mouth, esophogus, stomach, and other tissues with which contact is made, and may be fatal. May cause: headache. nausea. vomiting. May cause severe burns to the: digestive tract. Very large doses cause violent colic, diarrhea, depression and death.
Medical Conditions Aggravated by Exposure to Product: Asthma.
Other: SULFUR DIOXIDE GIVEN OFF BY THIS PRODUCT HAS BEEN SHOWN TO CAUSE BREATHING DIFFICULTIES IN ASTHMATICS. May cause severe allergic reaction in some asthmatics and sulfite sensitive individuals.
Cancer Information:
This product does not contain 0.1% or more of the known or potential carcinogens listed in NTP, IARC, or OSHA.,
SECTION 12. ECOLOGICAL INFORMATION
,Ecotoxicological Information: LC50 Fathead Minnow (96 hours): 319 ppm
Chemical Fate Information: No data available.,
SECTION 13. DISPOSAL CONSIDERATIONS
,Hazardous Waste Number: N.A.
Disposal Method: Dispose of in a permitted hazardous waste management facility following all local, state and federal regulations. If approved, neutralize material and flush to sewer. Since emptied containers retain product residue, follow label warnings even after container is emptied. DO NOT pressurize, cut, weld, solder, drill, grind or expose empty containers to heat, flame, sparks or other sources of ignition.,
SECTION 14. TRANSPORT INFORMATION
,D O T (Department of Transportation):
Identification Number: UN2693
Proper Shipping Name: BISULFITES, AQUEOUS SOLUTIONS, N.O.S (CONTAINS MAGNESIUM
BISULFITE)
Hazard Class: 8
Packing Group: III
Label Required: CORROSIVE,
SECTION 15. REGULATORY INFORMATION
,TSCA Inventory Status: This product or all components of this product are listed on the EPA/TSCA Inventory of Chemical Substances.
SARA Title III Section 311/312 Category Hazards:
Immediate (Acute)
Yes
Delayed (Chronic)
Yes
Fire Hazard
No
Pressure Release
No
Reactive
No
Regulated Components:
Component
No components found.
*Prop 65 - May Contain the Following Trace Components:
Sulfur Dioxide
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 See more Magnesium products.
See more Magnesium products. In its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. Commercially, magnesium is primarily used in the creation of strong and lightweight
In its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. Commercially, magnesium is primarily used in the creation of strong and lightweight 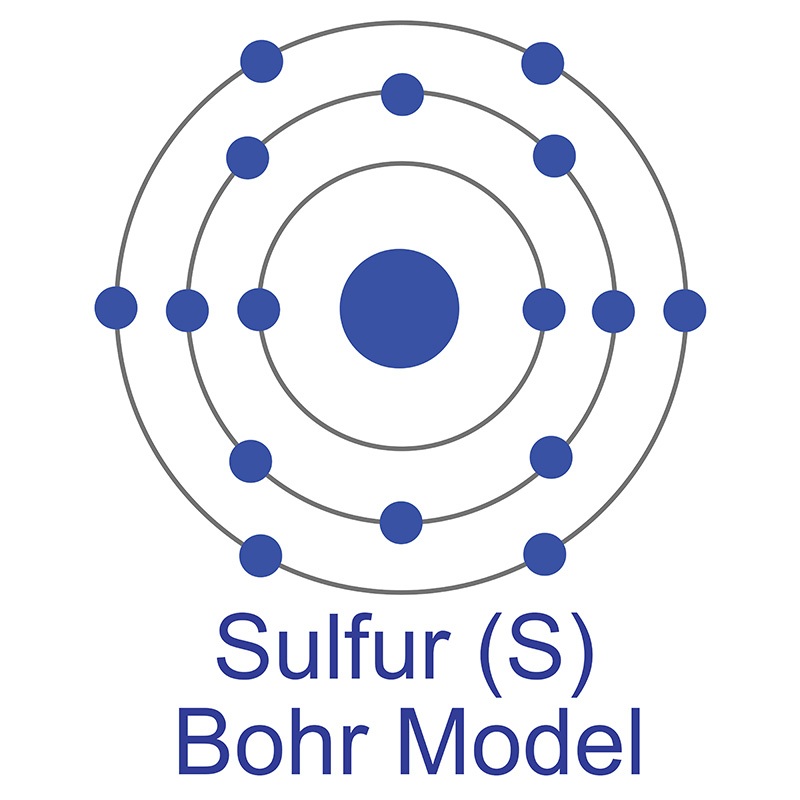 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.