About Ruthenium
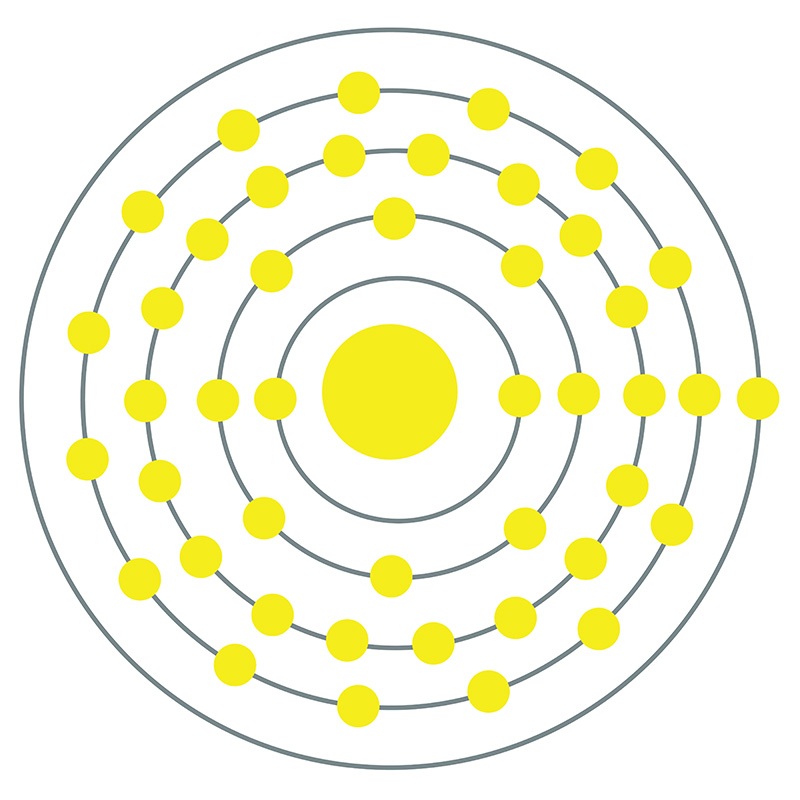
Ruthenium was discovered in 1844 by Karl Ernst Claus and named for Ruthenia, the Latin word for Claus’s native Rus. It was the last member of the platinum group of elements to be officially discovered, and like other members of that family is notable for being hard and corrosion resistant. In fact, ruthenium is harder than both platinum and palladium, and like the more expensive rhodium can be used to harden alloys of those elements. Thin-coatings of platinum-ruthenium and palladium-ruthenium alloys are often used to make electrical contacts wear-resistant. Additionally, ruthenium is added to titanium to impart corrosion resistance and used in high-temperature single-crystal superalloys used most commonly in aerospace applications. Ruthenium is also compatible with vapor deposition for producing thin films as well for use in other semiconductor processing techniques, and is under investigation for use in microelectronics.
In addition to the roles it plays in its metallic form, ruthenium finds many applications in the form of its compounds. Ruthenium dioxide and organometallic ruthenium complexes are used as catalysts in research, in organic and pharmaceutical chemistry, and in industrial settings. Ruthenium-based dyes have applications as biological stains in for light and electron microscopy, and are used in some dye-sensitized solar cells. The oils in fingerprints react with ruthenium tetroxide to produce darkly colored ruthenium dioxide, a process that is used to expose latent prints.
Finally, there are a few uses for ruthenium in medical applications. A radioactive isotope of ruthenium is used in radiotherapy of eye tumors, and several ruthenium-centered complexes are under investigation for anticancer properties.
Like other platinum group metals, ruthenium is typically obtained for commercial use as a byproduct from nickel and copper mining and processing, but can also be obtained from ores rich in platinum and from alluvial deposits. Along with rhodium and palladium, ruthenium is a decomposition product of uranium and could theoretically be recovered from spent nuclear fuel, but the problems inherent in working with radioactive materials make this an impractical source for the rare element.
Products
Ruthenium is primarily used for wear-resistant electrical contacts and the production of thick-film resistors. Ruthenium is one of the most effective hardeners for platinum and palladium and is alloyed with these metals to make electrical contacts for extremely wear resistant electronics and laboratory equipment. The corrosion resistance of titanium is improved a hundredfold by the addition of 0.1% ruthenium. Ruthenium is also a versatile catalyst. Hydrogen sulfide can be split catalytically by light using an aqueous suspension of cadmium sulfide particles loaded with ruthenium dioxide. Ruthenium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). 
 Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Ruthenium nanoparticles and nanopowders provide ultra-high surface area. Ruthenium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Ruthenium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Ruthenium nanoparticles and nanopowders provide ultra-high surface area. Ruthenium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Ruthenium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Ruthenium Properties
 Ruthenium is a Block D, Group 8, Period 5 element.
Ruthenium is a Block D, Group 8, Period 5 element. 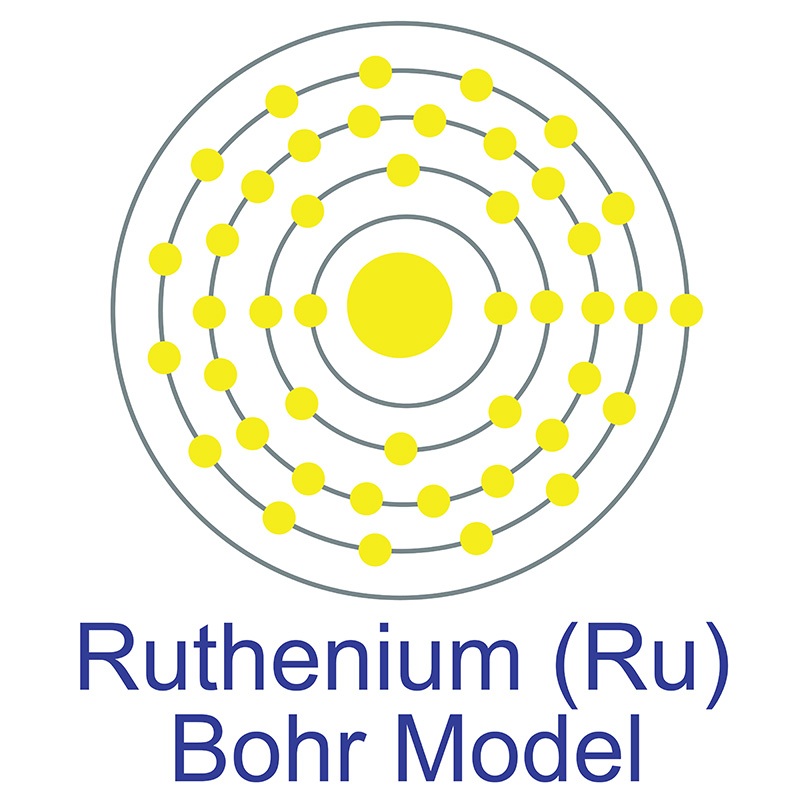 . It is found in pentlandite, pyroxenite, and platinum group metal ores. Ruthenium was first discovered by Karl Klaus in 1844. The name Ruthenium, originates from the Latin word 'Ruthenia' meaning Russia.
. It is found in pentlandite, pyroxenite, and platinum group metal ores. Ruthenium was first discovered by Karl Klaus in 1844. The name Ruthenium, originates from the Latin word 'Ruthenia' meaning Russia.
Health, Safety & Transportation Information for Ruthenium
Ruthenium in its elemental form is considered a carcinogen. Safety data for Ruthenium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Ruthenium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | 16-22-24/25 |
| RTECS Number | N/A |
| Transport Information | UN 3089 4.1/PG 2 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Ruthenium Isotopes
Naturally occurring ruthenium (Ru) has seven stable isotopes: 96Ru, 98Ru, 99Ru, 100Ru, 101Ru, 102Ru and 104Ru.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 87Ru | 86.94918(64)# | 50# ms [>1.5 µs] | ß+ to 87Tc | 1/2-# | N/A | 700.99 | - |
| 88Ru | 87.94026(43)# | 1.3(3) s [1.2(+3-2) s] | ß+ to 88Tc | 0+ | N/A | 717.45 | - |
| 89Ru | 88.93611(54)# | 1.38(11) s | ß+ to 89Tc | (7/2)(+#) | N/A | 729.26 | - |
| 90Ru | 89.92989(32)# | 11.7(9) s | ß+ to 90Tc | 0+ | N/A | 743.86 | - |
| 91Ru | 90.92629(63)# | 7.9(4) s | ß+ to 91Tc | (9/2+) | N/A | 754.73 | - |
| 92Ru | 91.92012(32)# | 3.65(5) min | ß+ to 92Tc | 0+ | N/A | 768.4 | - |
| 93Ru | 92.91705(9) | 59.7(6) s | ß+ to 93Tc | (9/2)+ | N/A | 779.27 | - |
| 94Ru | 93.911360(14) | 51.8(6) min | ß+ to 94Tc | 0+ | N/A | 792.94 | - |
| 95Ru | 94.910413(13) | 1.643(14) h | EC to 95Tc | 5/2+ | N/A | 801.95 | - |
| 96Ru | 95.907598(8) | Observationally Stable | - | 0+ | N/A | 812.83 | 27.83 |
| 97Ru | 96.907555(9) | 2.791(4) d | EC to 97Tc | 5/2+ | -0.78 | 820.91 | - |
| 98Ru | 97.905287(7) | STABLE | - | 0+ | N/A | 830.85 | 5.54 |
| 99Ru | 98.9059393(22) | STABLE | - | 5/2+ | -0.6413 | 838.93 | - |
| 100Ru | 99.9042195(22) | STABLE | - | 0+ | N/A | 847.94 | 1.87 |
| 101Ru | 100.9055821(22) | STABLE | - | 5/2+ | -0.7189 | 859.74 | 12.76 |
| 102Ru | 101.9043493(22) | STABLE | - | 0+ | N/A | 867.82 | 12.6 |
| 103Ru | 102.9063238(22) | 39.26(2) d | ß- to 103Rh | 3/2+ | 0.2 | 875.9 | 17.06 |
| 104Ru | 103.905433(3) | Observationally Stable | - | 0+ | N/A | 883.98 | 31.55 |
| 105Ru | 104.907753(3) | 4.44(2) h | ß- to 105Rh | 3/2+ | -0.3 | 892.06 | - |
| 106Ru | 105.907329(8) | 373.59(15) d | ß- to 106Rh | 0+ | N/A | 900.14 | 18.62 |
| 107Ru | 106.90991(13) | 3.75(5) min | ß- to 107Rh | (5/2)+ | N/A | 908.21 | - |
| 108Ru | 107.91017(12) | 4.55(5) min | ß- to 108Rh | 0+ | N/A | 906.98 | - |
| 109Ru | 108.91320(7) | 34.5(10) s | ß- to 109Rh | (5/2+)# | N/A | 915.06 | - |
| 110Ru | 109.91414(6) | 11.6(6) s | ß- to 110Rh | 0+ | N/A | 923.13 | - |
| 111Ru | 110.91770(8) | 2.12(7) s | ß- to 111Rh | (5/2+) | N/A | 931.21 | - |
| 112Ru | 111.91897(8) | 1.75(7) s | ß- to 112Rh | 0+ | N/A | 939.29 | - |
| 113Ru | 112.92249(8) | 0.80(5) s | ß- to 113Rh | (5/2+) | N/A | 938.05 | - |
| 114Ru | 113.92428(25)# | 0.53(6) s | ß- to 114Rh; ß- + n to 113Rh | 0+ | N/A | 946.13 | - |
| 115Ru | 114.92869(14) | 740(80) ms | ß- to 115Rh; ß- + n to 114Rh | N/A | N/A | 954.21 | - |
| 116Ru | 115.93081(75)# | 400# ms [>300 ns] | ß- to 116Rh | 0+ | N/A | 952.97 | - |
| 117Ru | 116.93558(75)# | 300# ms [>300 ns] | ß- to 117Rh | N/A | N/A | 961.05 | - |
| 118Ru | 117.93782(86)# | 200# ms [>300 ns] | ß- to 118Rh | 0+ | N/A | 969.13 | - |
| 119Ru | 118.94284(75)# | 170# ms [>300 ns] | Unknwon | N/A | N/A | 967.89 | - |
| 120Ru | 119.94531(86)# | 80# ms [>300 ns] | Unknwon | 0+ | N/A | 975.97 | - |