SECTION 1. IDENTIFICATION
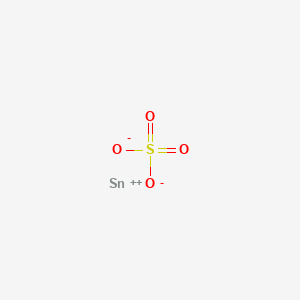
Product Name: Tin Sulfate Solution
Product Number: All applicable American Elements product codes, e.g. SN-SAT-02-SOL
, SN-SAT-03-SOL
, SN-SAT-04-SOL
, SN-SAT-05-SOL
CAS #: 7488-55-3
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
Classification (GHS-US)
Skin Corr. 1A H314
Carc. 1A H350


Signal word (GHS-US):
Danger
Hazard statements (GHS-US):
H314 - Causes severe skin burns and eye damage
H350 - May cause cancer
Precautionary statements (GHS-US):
P201 - Obtain special instructions before use
P202 - Do not handle until all safety precautions have been read and understood
P260 - Do not breathe mist/vapors/spray
P264 - Wash hand and other exposed areas thoroughly after handliong
P280 - Wear protective gloces/protective clothing/ eye protection/ face protection
P301+P330+P331 - IF SWALLOWED : rinse mouth. Do NOT induce vomiting
P303+P361+P353 - IF ON SKIN (or hair): Remove/ Take off immediately all contaminated clothing. Rinse skin with water/shower
P304+P340 - IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
P308+P313 - If exposed or concerned:Get medical advice/attention
P337+P313 - If eye irritation persists: Get medical advice/ attention
P363 - Wash contaminated clothing before reuse
Other Hazards
Strong inorganic acid mists containing sulfuric acid are classified as a known human carcinogen. This classification does not apply to sulfuric acid solutions.
Unknown acute toxicity (GHS-US)
None of the ingredients in the mixture are of unknown toxicity
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
N/A - product is a mixture
Mixture
Sulfuric Acid
CAS: 7664-93-9
0-2%
GHS-US
Skin Corr. 1A, H314
Carc. 1A, H350
Sulfuric Acid, Tin(II) Salt
CAS: 7488-55-3
15-25%
GHS-US
Not classified
SECTION 4. FIRST AID MEASURES
Swallowing:
Do NOT induce vomiting. Give large quantities of water. Call a physician. Do NOT neutralize the acid. Never give anything by mouth to an unconscious person.
Inhalation:
If inhaled, remove to fresh air immediately and have patient lie down if breathing is difficult. Call a physician.
Eyes/Skin:
IMMEDIATEDLY (within seconds) flush eyes or skin with plenty of water. Promptly get
medical help – apply compresses of iced water if there is a delay before medical treatment
SECTION 5. FIREFIGHTING MEASURES
Flashpoint (oF):
N/A
Flammable limits in air
LOWER: N/A UPPER: N/A
Extinguishing media:
Water, fog, foam
Special firefighting method:
Wear SCBA if fumes or mists are present
Unusual fire and explosion hazards:
Neutralize run-off with lime, soda ash, etc. Hydrogen gas formation is possible.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Steps to be taken if material is released or spilled:
Soak up small spills with sand, clay or distomoceous earth.
Neutralize large spills with lime or soda ash and transfer to a waste water treatment system.
Waste disposal method:
Cleaned-up material may be a RCRA hazardous waste on disposal. Do not flush to surface water or sanitary sewer system. Dispose of in accordance with all local, state, and federal regulations.
SECTION 7. HANDLING AND STORAGE
Precautions to be taken in handling and storage:
Keep out of sun and away from heat, sparks and flame.
Loosen closure carefully.
Other precautions:
Do not wash out container or use it for other purposes; replace closure after each withdrawal.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Sulfuric Acid, Tin(II) Salt
CAS: 7488-55-3
USA ACGIH
ACGIH TWA (mg/m3)
2mg/m3 (Tin)
USA OSHA
OSHA PEL (TWA) (mg/m3)
2mg/m3 (Tin)
Sulfuric Acid
CAS: 7664-93-9
USA ACGIH
ACGIH TWA (mg/m3)
0.2mg/m3
USA OSHA
OSHA PEL (TWA) (mg/m3)
1mg/m3
Appropriate Engineering Controls: Ensure tha eyewash station and safety showers are close to the workstation location.
Personal Protective equipment: Avoid all unnecessary exposure.
Hand Protection: Wear protective gloves.
Eye Protection: Chemical goggles or face shield
Skin and body protection: Wear suitable protective clothing.
Respiratory Protection: Not typically required if exposures are below established limits. If needed, use NIOS appproved respirator.
Other information: Do not eat, drink or smoke during use.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling Point (oF @ 760 mmHg): > 212
Specific gravity (H2O = 1 @ 72oF): 1.210
Solubility in water: Complete
Evaporation rate (butyl acetate = 1): > 1
SECTION 10. STABILITY AND REACTIVITY
Stability considerations/Conditions to avoid:
Stable / Water, organic materials (potential violent reaction, heat)
Hazardous polymerization/Conditions to avoid:
Will not occur / None
Incompatibility/Conditions to avoid:
Alkaline solutions, metals. Strong oxidizing, reducing or combustible organic materials.
Hazardous combustion or Decomposition products:
Hazardous gases may be generated on contact with cyanides, sulfides and carbides.
SECTION 11. TOXICOLOGICAL INFORMATION
Acute toxicity: Not classified
Sulfuric Acid
CAS: 7664-93-9
LD50 oral rat: 2140 mg/kg
LC50 inhalation rat: 510 mg/m3 (Exposure time:2 h)
Skin Corrosion/irritation: Causes severe skin burns and eye damage
Serious eye damage/irritation: Causes serious eye damage
Respiratory or Skin sentization: Not Classified
Germ cell mutagenicity: Not Classified
Carcinogenicity: May cause cancer in mist form
Sulfuric Acid
CAS: 7664-93-9
IARC group
1 - Carcinogenic to humans (only "strong inorganic acid mists containing sulfuric acid")
Reproductive toxicity: Not Classified
Specific target organ toxicity (single exposure): Not classified
Specific target organ toxicity (repeated exposure): Not classified
Aspiration hazard: Not classified
Potantial Adverse Human health effects and symptoms: No additonal infomation available
Symptoms/injuries If inhaled: May cause cancer by Inhalation
Symptoms/injuries In case of eye contact: causes serious eye irritation
SECTION 12. ECOLOGICAL INFORMATION
Toxicity
Sulfuric Acid
CAS: 7664-93-9
LC50 fish 1 > 500mg/l (Exposure time: 96 h - Species: Brachydanio rerio [static])
Persistence and degradability:
Not established
Bioaccumulative potential:
Stannous Sulfate Solution
Not established
Sulfuric Acid
BCF fish 1 (no bioaccumulation)
Mobility in soil:
No additional information available
Other adverse effects
Other information: Avoid release to the environment
SECTION 13. DISPOSAL CONSIDERATIONS
Waste disposal recommendations: Dispose in a safe manner in accordance with local, state, and federal regulations
Ecology - waste materials: Avoid release to the environment.
SECTION 14. TRANSPORT INFORMATION
Department of Transportation: Domestic Ground
Proper shipping name: Corrosive Liquid, N.O.S.
Hazard Class: 8
ID & Packing Group Number: UN 1760, PG III
ERG Guide Number: 154
SECTION 15. REGULATORY INFORMATION
SARA Title III Program Section 313 Supplier Notifica
tion. This product contains the following toxic
chemicals:
Chemical Name CAS Number Concentration
Sulfuric Acid 7664-93-9 < 20%
State Right-to-Know Programs
Pennsylvania: This product contains the following chemicals listed in PA Code Title 34,
Hazardous Substance List: Sulfuric Acid
California: This product contains the following compounds subject to the reporting and
labeling requirements of Proposition 65: None
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
 See more Tin products.
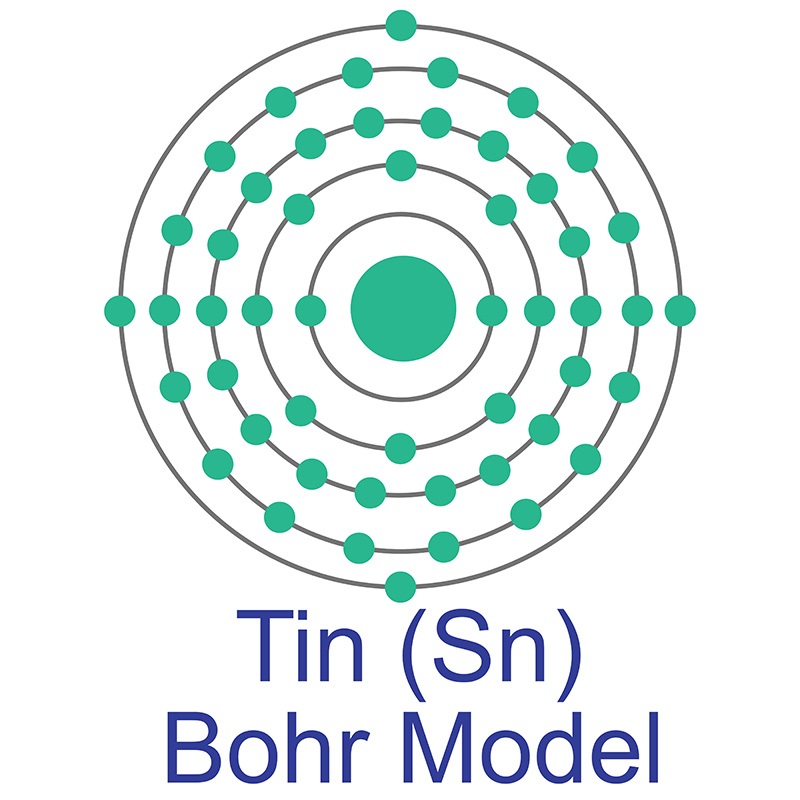
See more Tin products. Tin has nine stable isotopes and 18 unstable isotopes. Under 3.72 degrees Kelvin, Tin becomes a superconductor. Applications for tin include
Tin has nine stable isotopes and 18 unstable isotopes. Under 3.72 degrees Kelvin, Tin becomes a superconductor. Applications for tin include 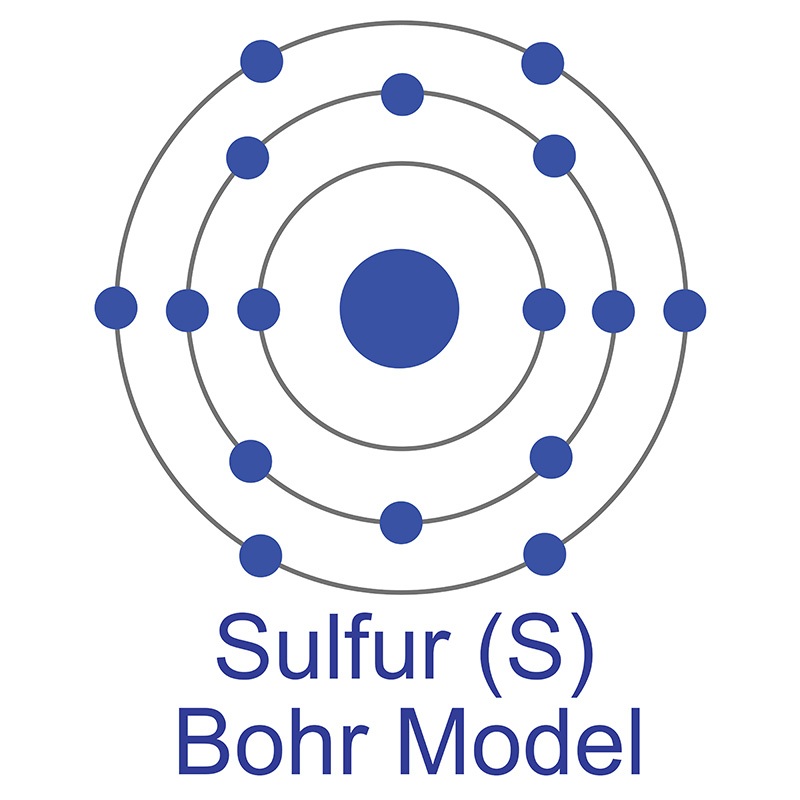 The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.
The number of electrons in each of Sulfur's shells is 2, 8, 6 and its electron configuration is [Ne] 3s2 3p4. In its elemental form, sulfur has a light yellow appearance. The sulfur atom has a covalent radius of 105 pm and a Van der Waals radius of 180 pm. In nature, sulfur can be found in hot springs, meteorites, volcanoes, and as galena, gypsum, and epsom salts. Sulfur has been known since ancient times but was not accepted as an element until 1777, when Antoine Lavoisier helped to convince the scientific community that it was an element and not a compound.