SECTION 1. IDENTIFICATION
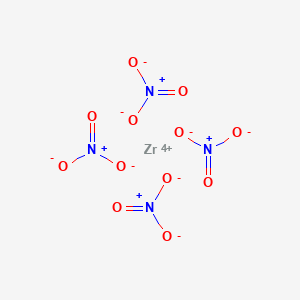
Product Name: Zirconium(IV) Nitrate
Product Number: All applicable American Elements product codes, e.g. ZR4-NAT-02
, ZR4-NAT-03
, ZR4-NAT-04
, ZR4-NAT-05
CAS #: 13746-89-9
Relevant identified uses of the substance: Scientific research and development
Supplier details:
American Elements
10884 Weyburn Ave.
Los Angeles, CA 90024
Tel: +1 310-208-0551
Fax: +1 310-208-0351
Emergency telephone number:
Domestic, North America: +1 800-424-9300
International: +1 703-527-3887
SECTION 2. HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Oxidizing. Corrosive.
Contact with combustible material may cause fire. Causes burns.
HMIS RATING
HEALTH: 3
FLAMMABILITY: 3
REACTIVITY: 0
NFPA RATING
HEALTH: 3
FLAMMABILITY: 3
REACTIVITY: 0
For additional information on toxicity, please refer to Section 11.
SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance Name CAS # SARA 313
ZIRCONIUM(IV) NITRATE 13746-89-9 No
Formula N4O12ZR
Synonyms Dusicnan zirkonicity (Czech) * Nitric acid,
zirconium(4+) salt (8CI,9CI) *
Tetranitratozirconium * Zirconium tetranitrate
RTECS Number: ZH8750000
SECTION 4. FIRST AID MEASURES
ORAL EXPOSURE
If swallowed, wash out mouth with water provided person is
conscious. Call a physician immediately.
INHALATION EXPOSURE
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
DERMAL EXPOSURE
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
EYE EXPOSURE
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
SECTION 5. FIREFIGHTING MEASURES
FLASH POINT
N/A
AUTOIGNITION TEMP
N/A
FLAMMABILITY
N/A
EXTINGUISHING MEDIA
Suitable: Carbon dioxide, dry chemical powder, or appropriate
foam.
Unsuitable: Do not use water.
FIREFIGHTING
Protective Equipment: Wear self-contained breathing apparatus
and protective clothing to prevent contact with skin and eyes.
Specific Hazard(s): Emits toxic fumes under fire conditions.
Contact with other material may cause fire.
Specific Method(s) of Fire Fighting: Use water spray to cool
fire-exposed containers.
SECTION 6. ACCIDENTAL RELEASE MEASURES
PROCEDURE TO BE FOLLOWED IN CASE OF LEAK OR SPILL
Evacuate area.
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear self-contained breathing apparatus, rubber boots, and heavy
rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Ventilate
area and wash spill site after material pickup is complete.
SECTION 7. HANDLING AND STORAGE
HANDLING
User Exposure: Do not breathe dust. Do not get in eyes, on skin,
on clothing. Avoid prolonged or repeated exposure.
STORAGE
Suitable: Keep tightly closed. Keep away from combustible
materials, heat, sparks, and open flame. Store in a cool dry
place.
SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
ENGINEERING CONTROLS
Safety shower and eye bath. Use only in a chemical fume hood.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory: Use respirators and components tested and approved
under appropriate government standards such as NIOSH (US) or CEN
(EU). Where risk assessment shows air-purifying respirators are
appropriate use a full-face particle respirator type N100 (US) or
type P3 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a
full-face supplied air respirator.
Hand: Compatible chemical-resistant gloves.
Eye: Chemical safety goggles.
GENERAL HYGIENE MEASURES
Wash contaminated clothing before reuse. Discard contaminated
shoes. Wash thoroughly after handling.
EXPOSURE LIMITS, RTECS
Country Source Type Value
USA ACGIH TWA 5 MG(ZR)/M3
STEL 10 MG(ZR)/M3
USA MSHA Standard-air TWA 5 MG(ZR)/M3
USA OSHA. PEL 8H TWA 5 MG(ZR)/M3
New Zealand OEL
Remarks: check ACGIH TLV
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance Physical State: Solid
Property Value At Temperature or Pressure
Molecular Weight 339,2000 AMU
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Freezing Point N/A
Vapor Pressure N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Bulk Density N/A
Odor Threshold N/A
Volatile% N/A
VOC Content N/A
Water Content N/A
Solvent Content N/A
Evaporation Rate N/A
Viscosity N/A
Surface Tension N/A
Partition Coefficient N/A
Decomposition Temp. N/A
Flash Point N/A
Explosion Limits N/A
Flammability N/A
Autoignition Temp N/A
Refractive Index N/A
Optical Rotation N/A
Miscellaneous Data N/A
Solubility N/A
N/A = not available
SECTION 10. STABILITY AND REACTIVITY
STABILITY
Stable: Unstable.
Materials to Avoid: Strong acids, Strong bases, Strong oxidizing
agents, Amines, Polymerizing initiators, Reducing agents, Finely
powdered metals.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Nitrogen oxides, Zirconium
oxides.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
SECTION 11. TOXICOLOGICAL INFORMATION
ROUTE OF EXPOSURE
Skin Contact: Causes burns.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: Causes burns.
Inhalation: May be harmful if inhaled. Material is extremely
destructive to the tissue of the mucous membranes and upper
respiratory tract.
Ingestion: May be harmful if swallowed.
SIGNS AND SYMPTOMS OF EXPOSURE
Inhalation may result in spasm, inflammation and edema of the
larynxand bronchi, chemical pneumonitis, and pulmonary edema.
Symptoms of exposure may include burning sensation, coughing,
wheezing, laryngitis, shortness of breath, headache, nausea, and
vomiting. To the best of our knowledge, the chemical, physical,
and toxicological properties have not been thoroughly
investigated. Material is extremely destructive to tissue of the
mucous membranes and upper respiratory tract, eyes, and skin.
TOXICITY DATA
Oral
Rat
2290,000000 mg/kg
LD50
ACGIH CARCINOGEN LIST
Rating: A4
SECTION 12. ECOLOGICAL INFORMATION
No data available.
SECTION 13. DISPOSAL CONSIDERATIONS
APPROPRIATE METHOD OF DISPOSAL OF SUBSTANCE OR PREPARATION
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
SECTION 14. TRANSPORT INFORMATION
DOT
Proper Shipping Name: Nitrates, inorganic, n.o.s.
UN#: 1477
Class: 5.1
Packing Group: Packing Group II
Hazard Label: Oxidizer
PIH: Not PIH
IATA
Proper Shipping Name: Nitrates, inorganic, n.o.s.
IATA UN Number: 1477
Hazard Class: 5.1
Packing Group: II
SECTION 15. REGULATORY INFORMATION
EU ADDITIONAL CLASSIFICATION
Symbol of Danger: O-C
Indication of Danger: Oxidizing. Corrosive.
R: 8-34
Risk Statements: Contact with combustible material may cause
fire. Causes burns.
S: 17-45-26-36/37/39
Safety Statements: Keep away from combustible material. In case
of accident or if you feel unwell, seek medical advice
immediately (show the label where possible). In case of contact
with eyes, rinse immediately with plenty of water and seek
medical advice. Wear suitable protective clothing, gloves, and
eye/face protection.
US CLASSIFICATION AND LABEL TEXT
Indication of Danger: Oxidizing. Corrosive.
Risk Statements: Contact with combustible material may cause
fire. Causes burns.
Safety Statements: Keep away from combustible material. In case
of accident or if you feel unwell, seek medical advice
immediately (show the label where possible). In case of contact
with eyes, rinse immediately with plenty of water and seek
medical advice. Wear suitable protective clothing, gloves, and
eye/face protection.
UNITED STATES REGULATORY INFORMATION
SARA LISTED: No
TSCA INVENTORY ITEM: Yes
CANADA REGULATORY INFORMATION
WHMIS Classification: This product has been classified in
accordance with the hazard criteria of the CPR, and the MSDS
contains all the information required by the CPR.
DSL: No
NDSL: Yes
SECTION 16. OTHER INFORMATION
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.

 The number of electrons in each of Zirconium's shells is 2, 8, 18, 10, 2 and its electron configuration is [Kr]4d2 5s2. The zirconium atom has a radius of 160 pm and a Van der Waals radius of 186 pm. Zirconium was discovered by Martin Heinrich Klaproth in 1789 and first isolated by Jöns Jakob Berzelius in 1824. In its elemental form, zirconium has a silvery white appearance that is similar to titanium. Zirconium's principal mineral is zircon (zirconium
The number of electrons in each of Zirconium's shells is 2, 8, 18, 10, 2 and its electron configuration is [Kr]4d2 5s2. The zirconium atom has a radius of 160 pm and a Van der Waals radius of 186 pm. Zirconium was discovered by Martin Heinrich Klaproth in 1789 and first isolated by Jöns Jakob Berzelius in 1824. In its elemental form, zirconium has a silvery white appearance that is similar to titanium. Zirconium's principal mineral is zircon (zirconium  Zirconium is commercially produced as a byproduct of
Zirconium is commercially produced as a byproduct of