About Terbium
In 1787, army-lieutenant and chemist Carl Axel Arrhenius found a rock in a quarry near the Swedish village of Ytterby which he suspected contained the newly discovered element tungsten. Analysis by other chemists did not bear out his suspicions, but ultimately four new elements were identified from Arrhenius’s ytterbite. Carl Gustaf Mosander isolated three new oxides in 1843, one of which he named terbia, giving the corresponding element the name terbium.
A variety of terbium-doped compounds can act as phosphors for use in television screens, other displays, lighting, or sensors. Most notably, terbium green phosphors are used in trichromatic fluorescent lamps along with europium blue and red phosphors to produce white light with greater energy efficiency than incandescent lighting. In zirconia oxide ceramics, terbium can act both as a light-emitting dopant and a crystal stabilizer, producing a material with excellent structural and optical properties. Other terbium doped compounds have useful electrical properties that make them potentially useful in the semiconductor industry.
Terfenol-D is an alloy of terbium, iron, and dysprosium that is magnetostrictive: it contracts or expands when exposed to magnetic fields. This property allows direct conversion between electrical and mechanical power, and the alloy is used in sensors, actuators, and acoustic and ultrasonic transducers, and active noise and vibration cancelling devices. Terbium was also a component of gadolinium-terbium-iron thin films used in magneto-optical memory storage devices and specialized compact discs (CD-MO), but other forms of computer memory are currently preferred for most applications. Additionally, engineered terbium-binding peptides are being developed for use as sensitive optical biosensors for sensing enzymatic activity, and several radioactive terbium isotopes are being developed for medical applications as diagnostic tracers or cancer treatments.
Terbium is a rare earth element and can be found in any rare earth containing mineral, but as a heavy rare earth element (HREE) it is more common in HREE-enriched minerals such as xenotime and euxenite. Additionally, terbium is present in ion adsorption clays, which are a major source of HREEs due to their relative ease of processing, despite the low percentage quantities of rare earths they contain.
Products
Terbium Properties
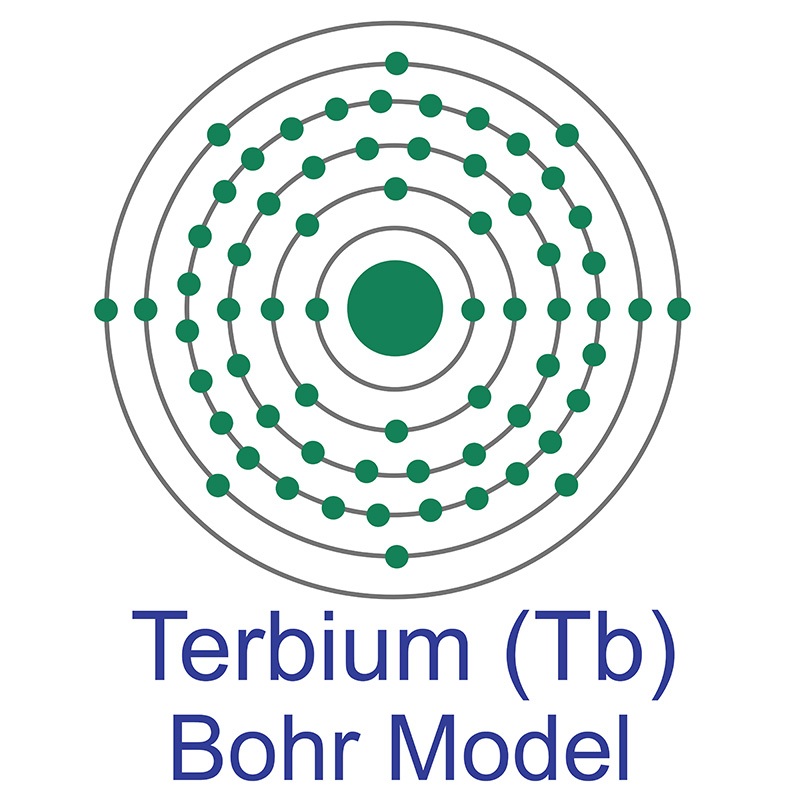 Terbium is a Block F, Group 3, Period 6 element. The number of electrons in each of Terbium's shells is 2, 8, 18, 27, 8, 2 and its electronic configuration is [Xe]4f9 6s2. The terbium atom has a radius of 177.pm and its Van der Waals radius is 221.pm. In its elemental form, CAS 7440-27-9, terbium has a silvery-white appearance. Terbium was discovered and first isolated by Carl Gustaf Mosander in 1842.
It is named after "Ytterby", the town in Sweden where it was discovered.
Terbium is a Block F, Group 3, Period 6 element. The number of electrons in each of Terbium's shells is 2, 8, 18, 27, 8, 2 and its electronic configuration is [Xe]4f9 6s2. The terbium atom has a radius of 177.pm and its Van der Waals radius is 221.pm. In its elemental form, CAS 7440-27-9, terbium has a silvery-white appearance. Terbium was discovered and first isolated by Carl Gustaf Mosander in 1842.
It is named after "Ytterby", the town in Sweden where it was discovered.
Health, Safety & Transportation Information for Terbium
Terbium is considered to be somewhat toxic. Safety data for Terbium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Terbium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Terbium Isotopes
Naturally occurring terbium (Tb) has one stable isotope: 159Tb
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 135Tb | 135 | 0.94(+33-22) ms | Unknown | (7/2-) | N/A | N/A | - |
| 136Tb | 135.96138(64)# | 0.2# s | Unknown | N/A | N/A | 1063.04 | - |
| 137Tb | 136.95598(64)# | 600# ms | Unknown | 11/2-# | N/A | 1080.43 | - |
| 138Tb | 137.95316(43)# | 800# ms [>200 ns] | ß+ to 138Gd; p to 137Gd | N/A | N/A | 1088.51 | - |
| 139Tb | 138.94829(32)# | 1.6(2) s | ß+ to 139Gd; ß+ + p to 139Eu | 11/2-# | N/A | 1105.9 | - |
| 140Tb | 139.94581(86) | 2.4(2) s | ß+ to 140Gd | 5 | N/A | 1113.98 | - |
| 141Tb | 140.94145(11) | 3.5(2) s | ß+ to 141Gd | (5/2-) | N/A | 1122.06 | - |
| 142Tb | 141.93874(32)# | 597(17) ms | ß+ to 142Gd; ß+ + p to 141Eu | 1+ | N/A | 1139.46 | - |
| 143Tb | 142.93512(6) | 12(1) s | ß+ to 143Gd | (11/2-) | N/A | 1147.54 | - |
| 144Tb | 143.93305(3) | ~1 s | ß+ to 144Gd | 1+ | N/A | 1155.61 | - |
| 145Tb | 144.92927(6) | 20# min | ß+ to 145Gd | (3/2+) | N/A | 1173.01 | - |
| 146Tb | 145.92725(5) | 8(4) s | ß+ to 146Gd | 1+ | N/A | 1181.09 | - |
| 147Tb | 146.924045(13) | 1.64(3) h | ß+ to 147Gd | 1/2+# | N/A | 1189.17 | - |
| 148Tb | 147.924272(15) | 60(1) min | ß+ to 148Gd | 2- | N/A | 1197.25 | - |
| 149Tb | 148.923246(5) | 4.118(25) h | ß+ to 149Gd; a to 145Eu | 1/2+ | N/A | 1205.32 | - |
| 150Tb | 149.923660(8) | 3.48(16) h | ß+ to 150Gd; a to 146Eu | (2-) | N/A | 1213.4 | - |
| 151Tb | 150.923103(5) | 17.609(1) h | ß+ to 151Gd; a to 147Eu | 1/2(+) | N/A | 1221.48 | - |
| 152Tb | 151.92407(4) | 17.5(1) h | ß+ to 152Gd; a to 148Eu | 2- | N/A | 1229.56 | - |
| 153Tb | 152.923435(5) | 2.34(1) d | EC to 153Gd | 5/2+ | 3.5 | 1237.64 | - |
| 154Tb | 153.92468(5) | 21.5(4) h | EC to 154Gd; ß- to 154Dy | 0(+#) | 0.9 | 1245.72 | - |
| 155Tb | 154.923505(13) | 5.32(6) d | EC to 155Gd | 3/2+ | 2 | 1253.8 | - |
| 156Tb | 155.924747(5) | 5.35(10) d | EC to 156Gd; ß- to 156Dy | 3- | 1.4 | 1261.87 | - |
| 157Tb | 156.9240246(27) | 71(7) y | EC to 157Gd | 3/2+ | 2 | 1269.95 | - |
| 158Tb | 157.9254131(28) | 180(11) y | EC to 158Gd; ß- to 158Dy | 3- | 1.76 | 1278.03 | - |
| 159Tb | 158.9253468(27) | STABLE | - | 3/2+ | 2.014 | 1286.11 | 100 |
| 160Tb | 159.9271676(27) | 72.3(2) d | ß- to 160Dy | 3- | 1.79 | 1294.19 | - |
| 161Tb | 160.9275699(28) | 6.906(19) d | ß- to 161Dy | 3/2+ | 2.2 | 1302.27 | - |
| 162Tb | 161.92949(4) | 7.60(15) min | ß- to 162Dy | 1- | N/A | 1310.35 | - |
| 163Tb | 162.930648(5) | 19.5(3) min | ß- to 163Dy | 3/2+ | N/A | 1309.11 | - |
| 164Tb | 163.93335(11) | 3.0(1) min | ß- to 164Dy | (5+) | N/A | 1317.19 | - |
| 165Tb | 164.93488(21)# | 2.11(10) min | ß- to 165Dy | 3/2+# | N/A | 1325.27 | - |
| 166Tb | 165.93799(11) | 25.6(22) s | ß- to 166Dy | N/A | N/A | 1333.35 | - |
| 167Tb | 166.94005(43)# | 19.4(27) s | ß- to 167Dy | 3/2+# | N/A | 1332.11 | - |
| 168Tb | 167.94364(54)# | 8.2(13) s | ß- to 168Dy | 4-# | N/A | 1340.19 | - |
| 169Tb | 168.94622(64)# | 2# s | ß- to 169Dy | 3/2+# | N/A | 1348.27 | - |
| 170Tb | 169.95025(75)# | 3# s | ß- to 170Dy | N/A | N/A | 1347.03 | - |
| 171Tb | 170.95330(86)# | 500# ms | ß- to 171Dy | 3/2+# | N/A | 1355.11 | - |
 Terbium is primarily used in phosphors, particularly in fluorescent lamps and as the high intensity green emitter used in projection televisions, such as the yttrium-aluminum-garnet (Tb:YAG) variety. Terbium
Terbium is primarily used in phosphors, particularly in fluorescent lamps and as the high intensity green emitter used in projection televisions, such as the yttrium-aluminum-garnet (Tb:YAG) variety. Terbium 
