Rare Earths: Overview
The rare earth elements (REEs) are a group of seventeen chemical elements on the periodic table: scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. The rare earths exhibit unique chemical properties that make them highly valued in a wide variety of applications. Despite their name, with the exception of Promethium, which has no stable isotopes, the rare earths are not scarce on earth in absolute terms. Their name is derived from the rarity of the minerals in which they found in high enough concentration to be economically extracted.
For most of the years since their earliest discovery in the late eighteenth century, the existence of a few small mines exploiting these rare deposits was sufficient to meet global demand for these elements at any given time. In the last two decades, most of the already scarce REE mines around the world ceased operation in response to low cost rare earth exports from China. However, as rare earth products are becoming increasingly critical raw materials for large sectors of the world economy, it has become clear that counting on the supply of this essential resource by a single nation carries significant risks. Today this recognition has spurred renewed interest in discovery and development of rare earth sources in outside of China.
Rare Earth Minerals
The following types of mineral deposits comprise the primary sources of rare earth elements:
Bastnäsite

A carbonate-floride mineral that always contains rare earth elements. The generalized formula for this mineral is (Ce,La,Y)CO3F, but it can be more accurately described as three subtypes of minerals: bastnäsite-(Ce), bastnäsite-(La), and bastnäsite-(Y), which have greater proportions of cerium, lanthanum, and yttrium respectively. Most naturally-occurring bastnäsite is bastnäsite-(Ce). Praseodymium typically accounts for 5% of the mineral's element composition. Bastnäsite is a major source of the light rare earth elements, and most known major deposits are in the United States and China.
Monazite
A phosphate mineral that always contains rare earth metals. It has the generalized formula (Ce,La)PO4, but like bastnäsite, it is found in several forms, each named for its most abundant rare earth constituent: monazite-(Ce) , monazite-(La), monazite-(Nd) , and monazite-(Sm). Each of these minerals contains its named rare earth element in addition to several others in smaller proportions. Monazite-(Ce) is by far the most common, and has the formula (Ce,La,Nd,Th)(PO4). Monazite is a major source of the light rare earth elements and thorium. Due to the presence of thorium and small amounts of uranium, monazite is typically radioactive.

Xenotime
A phosphate mineral whose major component is yttrium orthophosphate (YPO4). It may contain dysprosium, erbium, terbium, thorium, ytterbium,and uranium as secondary components, and as such is a significant source of the heavy rare earth elements.
Lateritic Ion-Adsorption Clays
These deposits contain all of the rare earth elements in varying proportions, and can be processed to concentrate these elements with much greater ease than other types of rare earth minerals. This relative ease of processing is key to the value of these clays, as their percentage composition of REEs is low compared to the rare earth minerals discussed above. Deposits of these clays in southern China are important sources for REEs.
Rare Earth Elements
 Cerium is the most abundant of the rare earths. It is
characterized chemically by having two valence states , the +3 cerous and +4 ceric states. The ceric state
is the only non-trivalent rare earth ion stable in aqueous solutions, making it strongly acidic and
moderately toxic, as well as a strong oxidizer.The cerous state closely resembles the other trivalent rare
earths. The numerous commercial applications for cerium include metallurgy, glass and glass polishing,
ceramics, catalysts, and in phosphors. In steel manufacturing it is used to remove free oxygen and sulfur by
forming stable oxysulfides and by tying up undesirable trace elements such as lead and
antimony. It is considered to be the most efficient glass polishing agent for
precision optical polishing. It is also used to decolor glass by keeping iron in its
ferrous state. The ability of cerium-doped glass to block out ultra
violet light is utilized in the manufacturing of medical glassware and aerospace windows; it is also used to
prevent polymers from darkening in sunlight and to suppress discoloration of television glass. It is applied
to optical components to improve performance. Cerium is used in a variety of ceramics iincluding dental compositions and as a phase stabilizer in zirconia-based products. Ceria
plays several catalytic roles: in catalytic converters, it acts as a stabilizer for the high surface area alumina, as a promoter of the water-gas shift reaction, as an oxygen
storage component and as an enhancer of the NOX reduction capability of rhodium.
Cerium is added to the dominant catalyst for the production of styrene from ethylbenezene to improve styrene
formation. It is used in FCC catalysts containing zeolites to provide both catalytic reactivity in the
reactor and thermal stability in the regenerator. American Elements produces cerium as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra high purity); metals in the form of foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
Cerium is the most abundant of the rare earths. It is
characterized chemically by having two valence states , the +3 cerous and +4 ceric states. The ceric state
is the only non-trivalent rare earth ion stable in aqueous solutions, making it strongly acidic and
moderately toxic, as well as a strong oxidizer.The cerous state closely resembles the other trivalent rare
earths. The numerous commercial applications for cerium include metallurgy, glass and glass polishing,
ceramics, catalysts, and in phosphors. In steel manufacturing it is used to remove free oxygen and sulfur by
forming stable oxysulfides and by tying up undesirable trace elements such as lead and
antimony. It is considered to be the most efficient glass polishing agent for
precision optical polishing. It is also used to decolor glass by keeping iron in its
ferrous state. The ability of cerium-doped glass to block out ultra
violet light is utilized in the manufacturing of medical glassware and aerospace windows; it is also used to
prevent polymers from darkening in sunlight and to suppress discoloration of television glass. It is applied
to optical components to improve performance. Cerium is used in a variety of ceramics iincluding dental compositions and as a phase stabilizer in zirconia-based products. Ceria
plays several catalytic roles: in catalytic converters, it acts as a stabilizer for the high surface area alumina, as a promoter of the water-gas shift reaction, as an oxygen
storage component and as an enhancer of the NOX reduction capability of rhodium.
Cerium is added to the dominant catalyst for the production of styrene from ethylbenezene to improve styrene
formation. It is used in FCC catalysts containing zeolites to provide both catalytic reactivity in the
reactor and thermal stability in the regenerator. American Elements produces cerium as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra high purity); metals in the form of foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
 Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. While
it has one of the highest magnetic moments of any of the rare earths (10.6µB), this has not resulted
in an ability to perform on its own as a practical alternative to neodymium
compositions. It is however now an essential additive in NdFeB production. It is also used in special
ceramic compositions based on barium titanate (BaTiO)
formulations. Recent research has examined the use of dysprosium in dysprosium-iron-garnet (DyIG) and silicon implanted with dysprosium and holmium to form donor
centers. Dysprosium is added to various advanced optical formulations
due to the fact that it emits in the 470-500 and 570-600 nm wavelengths. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. While
it has one of the highest magnetic moments of any of the rare earths (10.6µB), this has not resulted
in an ability to perform on its own as a practical alternative to neodymium
compositions. It is however now an essential additive in NdFeB production. It is also used in special
ceramic compositions based on barium titanate (BaTiO)
formulations. Recent research has examined the use of dysprosium in dysprosium-iron-garnet (DyIG) and silicon implanted with dysprosium and holmium to form donor
centers. Dysprosium is added to various advanced optical formulations
due to the fact that it emits in the 470-500 and 570-600 nm wavelengths. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
 Erbium has application in glass coloring, as an amplifier in
fiber optics, and in lasers for medical and dental use. The ion has a very narrow absorption band, coloring erbium
salts pink; iIt is therefore used in eyeware and decorative glassware. It can neutralize discoloring
impurities such as ferric ions and produce a neutral gray shade and s used in a
variety of glass products for this purpose. It is particularly useful
as an amplifier for fiber optic data transfer as it lases at the wavelength required to provide an efficient
optical method of amplification, 1.55 microns. Lasers based on Er:YAG are ideally suited for surgical
applications because of its ability to deliver energy without thermal build-up in tissue. Erbium is
available as metal and compounds with purities from 99% to 99.999% (ACS
grade to ultra-high purity); metals in the form of foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
Erbium has application in glass coloring, as an amplifier in
fiber optics, and in lasers for medical and dental use. The ion has a very narrow absorption band, coloring erbium
salts pink; iIt is therefore used in eyeware and decorative glassware. It can neutralize discoloring
impurities such as ferric ions and produce a neutral gray shade and s used in a
variety of glass products for this purpose. It is particularly useful
as an amplifier for fiber optic data transfer as it lases at the wavelength required to provide an efficient
optical method of amplification, 1.55 microns. Lasers based on Er:YAG are ideally suited for surgical
applications because of its ability to deliver energy without thermal build-up in tissue. Erbium is
available as metal and compounds with purities from 99% to 99.999% (ACS
grade to ultra-high purity); metals in the form of foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
 Europium is utilized primarily for its unique luminescent
behavior. Excitation of the europium atom by absorption of ultra violet radiation can result in specific
energy level transitions within the atom, creating an emission of visible radiation .In energy efficient
fluorescent lighting, europium oxide provides not only the necessary
red but also the blue. Several commercial blue phosphors are based on europium. Its luminesence is also
valuable in medical, surgical and biochemical applications. Europium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
Europium is utilized primarily for its unique luminescent
behavior. Excitation of the europium atom by absorption of ultra violet radiation can result in specific
energy level transitions within the atom, creating an emission of visible radiation .In energy efficient
fluorescent lighting, europium oxide provides not only the necessary
red but also the blue. Several commercial blue phosphors are based on europium. Its luminesence is also
valuable in medical, surgical and biochemical applications. Europium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
 Gadolinium is utilized for both its high magnetic moment
(7.94µB) and as a phosphor and scintillator material. When complexed with EDTA ligands, it is used as
an injectable contrast agent for patients undergoing magnetic resonance imaging. With its high magnetic
moment, gadolinium can reduce relaxation times and thereby enhance signal intensity. The extra stable
half-full 4f electron shell with no low lying energy levels creates applications as an inert phosphor host.
Gadolinium can therefore act as hosts for x-ray cassettes and in scintillator materials for computer
tomography. Gadolinium is available as metal and compounds with
purities from 99% to 99.999% (ACS grade to ultra-high
purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
Gadolinium is utilized for both its high magnetic moment
(7.94µB) and as a phosphor and scintillator material. When complexed with EDTA ligands, it is used as
an injectable contrast agent for patients undergoing magnetic resonance imaging. With its high magnetic
moment, gadolinium can reduce relaxation times and thereby enhance signal intensity. The extra stable
half-full 4f electron shell with no low lying energy levels creates applications as an inert phosphor host.
Gadolinium can therefore act as hosts for x-ray cassettes and in scintillator materials for computer
tomography. Gadolinium is available as metal and compounds with
purities from 99% to 99.999% (ACS grade to ultra-high
purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
 Holmium has the highest magnetic moment (10.6µB) of any
naturally occurring element. Because of this it has been used to create the highest known magnetic fields by
placing it within high strength magnets as a pole piece or magnetic flux concentrator. This magnetic
property also has value in yttrium-iron-garnet (YIG) lasers
for microwave equipment. Holmium lases at a human eye safe 2.08 microns ,allowing its use in a variety of
medical and dental applications in both yttrium-aluminum-garnet (YAG) and yttrium-lithium-fluoride (YLF) solid state lasers. The
wavelength allows for use in silica fibers designed for shorter wavelengths while still providing the
cutting strength of longer wave length equipment. Holmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
Holmium has the highest magnetic moment (10.6µB) of any
naturally occurring element. Because of this it has been used to create the highest known magnetic fields by
placing it within high strength magnets as a pole piece or magnetic flux concentrator. This magnetic
property also has value in yttrium-iron-garnet (YIG) lasers
for microwave equipment. Holmium lases at a human eye safe 2.08 microns ,allowing its use in a variety of
medical and dental applications in both yttrium-aluminum-garnet (YAG) and yttrium-lithium-fluoride (YLF) solid state lasers. The
wavelength allows for use in silica fibers designed for shorter wavelengths while still providing the
cutting strength of longer wave length equipment. Holmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
 Lanthanum is the first element in the rare earth or lanthanide
series. It is the model for all the other trivalent rare earths. After cerium, it is
the second most abundant of the rare earths. Lanthanum-rich lanthanide compositions have been used
extensively for cracking reactions in FCC catalysts, especially to manufacture low-octane fuel for heavy
crude oil. Lantahanum is found in monazite and bastnasite. The name Lanthanum originates from the Greek word
Lanthaneia which means 'To lie hidden'. It is utilized in green phosphors based on the aluminate
(La0.4Ce0.45Tb0.15)PO4. Lanthanide zirconates and lanthanum
strontium manganites are used for their catalytic and conductivity properties and lanthanum stabilized
zirconia has useful electrical and mechanical properties. Lanthanum's ability to bind with phosphates in
water creates numerous uses in water treatment. It is utilized
in laser crystals based on the yttrium-lanthanum-fluoride (YLF) composition. Lanthanum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
Lanthanum is the first element in the rare earth or lanthanide
series. It is the model for all the other trivalent rare earths. After cerium, it is
the second most abundant of the rare earths. Lanthanum-rich lanthanide compositions have been used
extensively for cracking reactions in FCC catalysts, especially to manufacture low-octane fuel for heavy
crude oil. Lantahanum is found in monazite and bastnasite. The name Lanthanum originates from the Greek word
Lanthaneia which means 'To lie hidden'. It is utilized in green phosphors based on the aluminate
(La0.4Ce0.45Tb0.15)PO4. Lanthanide zirconates and lanthanum
strontium manganites are used for their catalytic and conductivity properties and lanthanum stabilized
zirconia has useful electrical and mechanical properties. Lanthanum's ability to bind with phosphates in
water creates numerous uses in water treatment. It is utilized
in laser crystals based on the yttrium-lanthanum-fluoride (YLF) composition. Lanthanum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
 Lutetium is the last member of the rare earth series. Unlike
most rare earths, it lacks a magnetic moment; it also has the smallest metallic radius of any rare earth. It
is perhaps the least naturally abundant of the lanthanides. It is the ideal host for x-ray phosphors because
it produces the densest known white material, lutetium tantalate (LuTaO4). It
is utilized as a dopant in matching lattice parameters of certain substrate garnet crystals, such as
indium-gallium-garnet (IGG) crystals due its lack of a magnetic moment. Lutetium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
Lutetium is the last member of the rare earth series. Unlike
most rare earths, it lacks a magnetic moment; it also has the smallest metallic radius of any rare earth. It
is perhaps the least naturally abundant of the lanthanides. It is the ideal host for x-ray phosphors because
it produces the densest known white material, lutetium tantalate (LuTaO4). It
is utilized as a dopant in matching lattice parameters of certain substrate garnet crystals, such as
indium-gallium-garnet (IGG) crystals due its lack of a magnetic moment. Lutetium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
 Neodymium is the most abundant of the rare earths after cerium and lanthanum. Primary applications include lasers, glass coloring and tinting, dielectrics and, most importantly, as the
fundamental basis for neodymium-iron-boron permanent
magnets. Neodymium has a strong absorption band centered at 580 nm, which is very close to the human eye's
maximum level of sensitivity making it useful in protective lenses for welding goggles. It is also used in
CRT displays to enhance contrast between reds and greens and highly valued in glass manufacturing for its attractive purple coloring. Neodymium is
included in many formulations of barium titanate, used as
dielectric coatings and in multi-layer capacitors essential to electronic equipment. Neodymium is available
as metal and compounds with purities from 99% to 99.999% (ACS grade
to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
Neodymium is the most abundant of the rare earths after cerium and lanthanum. Primary applications include lasers, glass coloring and tinting, dielectrics and, most importantly, as the
fundamental basis for neodymium-iron-boron permanent
magnets. Neodymium has a strong absorption band centered at 580 nm, which is very close to the human eye's
maximum level of sensitivity making it useful in protective lenses for welding goggles. It is also used in
CRT displays to enhance contrast between reds and greens and highly valued in glass manufacturing for its attractive purple coloring. Neodymium is
included in many formulations of barium titanate, used as
dielectric coatings and in multi-layer capacitors essential to electronic equipment. Neodymium is available
as metal and compounds with purities from 99% to 99.999% (ACS grade
to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and compounds as submicron and nanopowder.
 Praseodymium resembles the typical trivalent rare earths;
however, it will exhibit a +4 state when stabilized in a zirconia
host. The element is found in most all light rare earth derivatives. It is highly valued in glass and ceramic production as a bright yellow pigment because of its
optimum reflectance at 560 nm. Much research is being done on its optical properties for use in
amplification of telecommunication systems, including as a doping agent in fluoride fibers. Praseodymium
doped zirconia is a potential cathode for low temperature solid oxide
fuel cell (SOFC) applications. It is also used in the scintillator for medical CAT scans. Praseodymium
is available as metal and compounds with purities from 99% to
99.999% (ACS grade to ultra-high purity); metals in the
form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
Praseodymium resembles the typical trivalent rare earths;
however, it will exhibit a +4 state when stabilized in a zirconia
host. The element is found in most all light rare earth derivatives. It is highly valued in glass and ceramic production as a bright yellow pigment because of its
optimum reflectance at 560 nm. Much research is being done on its optical properties for use in
amplification of telecommunication systems, including as a doping agent in fluoride fibers. Praseodymium
doped zirconia is a potential cathode for low temperature solid oxide
fuel cell (SOFC) applications. It is also used in the scintillator for medical CAT scans. Praseodymium
is available as metal and compounds with purities from 99% to
99.999% (ACS grade to ultra-high purity); metals in the
form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
 Promethium is the only radioactive member of the rare earths,
and the last to be discovered. The existence of an unknown element with atomic number 61 was predicted in
1914 by Henry Mosley, but the metal was not successfully produced and fully characterized until 1945 by
Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell at the Oak Ridge National Laboratory. The
name was derived from, Prometheus the Titan in Greek mythology who stole fire from Mount Olympus to give to
humans. All of promethium’s isotopes are radioactive and have short half-lives,
so appreciable amounts are not found in natural sources. Since the element can only be obtained from a lab,
it has few applications. The only isotope of promethium used outside of research is promethium-147, which is
produced and used in very small (milligram) quantities in atomic batteries and signal lights containing
phosphors that emit light in response to absorption of radiation emitted by the isotope. Since this
relatively stable isotope emits x-rays, it could theoretically be used for portable x-ray sources.
Promethium is the only radioactive member of the rare earths,
and the last to be discovered. The existence of an unknown element with atomic number 61 was predicted in
1914 by Henry Mosley, but the metal was not successfully produced and fully characterized until 1945 by
Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell at the Oak Ridge National Laboratory. The
name was derived from, Prometheus the Titan in Greek mythology who stole fire from Mount Olympus to give to
humans. All of promethium’s isotopes are radioactive and have short half-lives,
so appreciable amounts are not found in natural sources. Since the element can only be obtained from a lab,
it has few applications. The only isotope of promethium used outside of research is promethium-147, which is
produced and used in very small (milligram) quantities in atomic batteries and signal lights containing
phosphors that emit light in response to absorption of radiation emitted by the isotope. Since this
relatively stable isotope emits x-rays, it could theoretically be used for portable x-ray sources.
 Samarium is primarily utilized in the production of samarium-cobalt (Sm2Co17) permanent
magnets, which replaced the more expensive platinum-cobalt
magnets in the early 1970s. While now overshadowed by the less expensive neodymium-iron-boron magnets, they are still valued for their ability to
function at high temperatures. They are utilized in lightweight electronic equipment where size or space is
a limiting factor and where functionality at high temperature is a concern. Applications include electronic
watches, aeospace equipment, microwave technology and servomotors. Because of its weak spectral absorption
band samarium is used in the filter glass on Nd:YAG
solid state lasers to surround the laser rod to improve efficiency by absorbing stray emissions. Samarium
forms stable titanate compounds with useful dielectric properties suitable for coatings and in capacitors at
microwave frequencies. It is also used in laser applications, and for its dielectric properties. Samarium is
available as metal and compounds with purities from 99% to 99.999%
(ACS grade to ultra-high purity); metals in the form of
foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
Samarium is primarily utilized in the production of samarium-cobalt (Sm2Co17) permanent
magnets, which replaced the more expensive platinum-cobalt
magnets in the early 1970s. While now overshadowed by the less expensive neodymium-iron-boron magnets, they are still valued for their ability to
function at high temperatures. They are utilized in lightweight electronic equipment where size or space is
a limiting factor and where functionality at high temperature is a concern. Applications include electronic
watches, aeospace equipment, microwave technology and servomotors. Because of its weak spectral absorption
band samarium is used in the filter glass on Nd:YAG
solid state lasers to surround the laser rod to improve efficiency by absorbing stray emissions. Samarium
forms stable titanate compounds with useful dielectric properties suitable for coatings and in capacitors at
microwave frequencies. It is also used in laser applications, and for its dielectric properties. Samarium is
available as metal and compounds with purities from 99% to 99.999%
(ACS grade to ultra-high purity); metals in the form of
foil, sputtering target,
and rod, and compounds as submicron and nanopowder.
 Terbium is primarily used in phosphors, particularly in
fluorescent lamps and as the high intensity green emitter used in projection televisions, such as the
yttrium-aluminum-garnet (Tb:YAG) variety. Terbium responds efficiently in x-ray excitation and is,
therefore, used as an x-ray phosphor. Terbium alloys are also used in
magneto-optic recording films, such as Tb-Fe-Co. A variety of terbium-doped compounds can act as phosphors
for use in television screens, other displays, lighting, or sensors. Most notably, terbium green phosphors are used in trichromatic fluorescent lamps along with europium
blue and red phosphors to produce white light with greater energy efficiency than incandescent lighting. In
zirconia oxide ceramics, terbium can act both as a light-emitting
dopant and a crystal stabilizer, producing a material with excellent structural and optical properties.
Other terbium doped compounds have useful electrical properties that make them potentially useful in the
semiconductor industry. Terfenol-D is an alloy of terbium, iron, and dysprosium
that is magnetostrictive: it contracts or expands when exposed to magnetic fields. This property allows
direct conversion between electrical and mechanical power, and the alloy is used in sensors, actuators, and
acoustic and ultrasonic transducers, and active noise and vibration cancelling devices. Terbium is available
as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
Terbium is primarily used in phosphors, particularly in
fluorescent lamps and as the high intensity green emitter used in projection televisions, such as the
yttrium-aluminum-garnet (Tb:YAG) variety. Terbium responds efficiently in x-ray excitation and is,
therefore, used as an x-ray phosphor. Terbium alloys are also used in
magneto-optic recording films, such as Tb-Fe-Co. A variety of terbium-doped compounds can act as phosphors
for use in television screens, other displays, lighting, or sensors. Most notably, terbium green phosphors are used in trichromatic fluorescent lamps along with europium
blue and red phosphors to produce white light with greater energy efficiency than incandescent lighting. In
zirconia oxide ceramics, terbium can act both as a light-emitting
dopant and a crystal stabilizer, producing a material with excellent structural and optical properties.
Other terbium doped compounds have useful electrical properties that make them potentially useful in the
semiconductor industry. Terfenol-D is an alloy of terbium, iron, and dysprosium
that is magnetostrictive: it contracts or expands when exposed to magnetic fields. This property allows
direct conversion between electrical and mechanical power, and the alloy is used in sensors, actuators, and
acoustic and ultrasonic transducers, and active noise and vibration cancelling devices. Terbium is available
as metal and compounds with purities from 99% to 99.999% (ACS grade to
ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
 Thulium is representative of the other lanthanides (rare
earths), similar in chemistry to yttrium. Notably, it is used as a dopant for garnets used as laser gain media, and such lasers are used in medical laser
applications such as laser surgery as well as in industrial and military applications. The radioactive isotope thulium-170 can be produced in nuclear reactors and is found in portable
x-ray devices used for medical diagnostics and manufacturing quality-control applications. Additionally,
this isotope can be used for radiotherapy cancer treatment. Thulium may be also used in high-temperature
superconductors, magnetic ceramic materials, personal radiation dosimeters, and phosphors for use in
anti-counterfeiting features of modern currency notes such as the Euro. Thulium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
Thulium is representative of the other lanthanides (rare
earths), similar in chemistry to yttrium. Notably, it is used as a dopant for garnets used as laser gain media, and such lasers are used in medical laser
applications such as laser surgery as well as in industrial and military applications. The radioactive isotope thulium-170 can be produced in nuclear reactors and is found in portable
x-ray devices used for medical diagnostics and manufacturing quality-control applications. Additionally,
this isotope can be used for radiotherapy cancer treatment. Thulium may be also used in high-temperature
superconductors, magnetic ceramic materials, personal radiation dosimeters, and phosphors for use in
anti-counterfeiting features of modern currency notes such as the Euro. Thulium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering
target, and rod, and componds as submicron and nanopowder.
 Ytterbium is being applied to numerous fiber amplifier and fiber
optic technologies and in various lasing applications. It has a single dominant absorption band at 985 in
the infrared range, making it useful in silicon photocells to directly convert radiant
energy to electricity. Ytterbium metal increases its electrical resistance when subjected to very high
stresses. This property is used in stress gauges for monitoring ground deformations from earthquakes and
nuclear explosions. It is also used in thermal barrier system bond coatings on nickel,
iron, and other transitional metal alloy substrates.
Ytterbium is available as metal and compounds with purities from 99%
to 99.999% (ACS grade to ultra-high purity); metals in
the form of foil, sputtering target, and rod, and componds as submicron and nanopowder.
Ytterbium is being applied to numerous fiber amplifier and fiber
optic technologies and in various lasing applications. It has a single dominant absorption band at 985 in
the infrared range, making it useful in silicon photocells to directly convert radiant
energy to electricity. Ytterbium metal increases its electrical resistance when subjected to very high
stresses. This property is used in stress gauges for monitoring ground deformations from earthquakes and
nuclear explosions. It is also used in thermal barrier system bond coatings on nickel,
iron, and other transitional metal alloy substrates.
Ytterbium is available as metal and compounds with purities from 99%
to 99.999% (ACS grade to ultra-high purity); metals in
the form of foil, sputtering target, and rod, and componds as submicron and nanopowder.
Rare Earth Applications
Catalysis
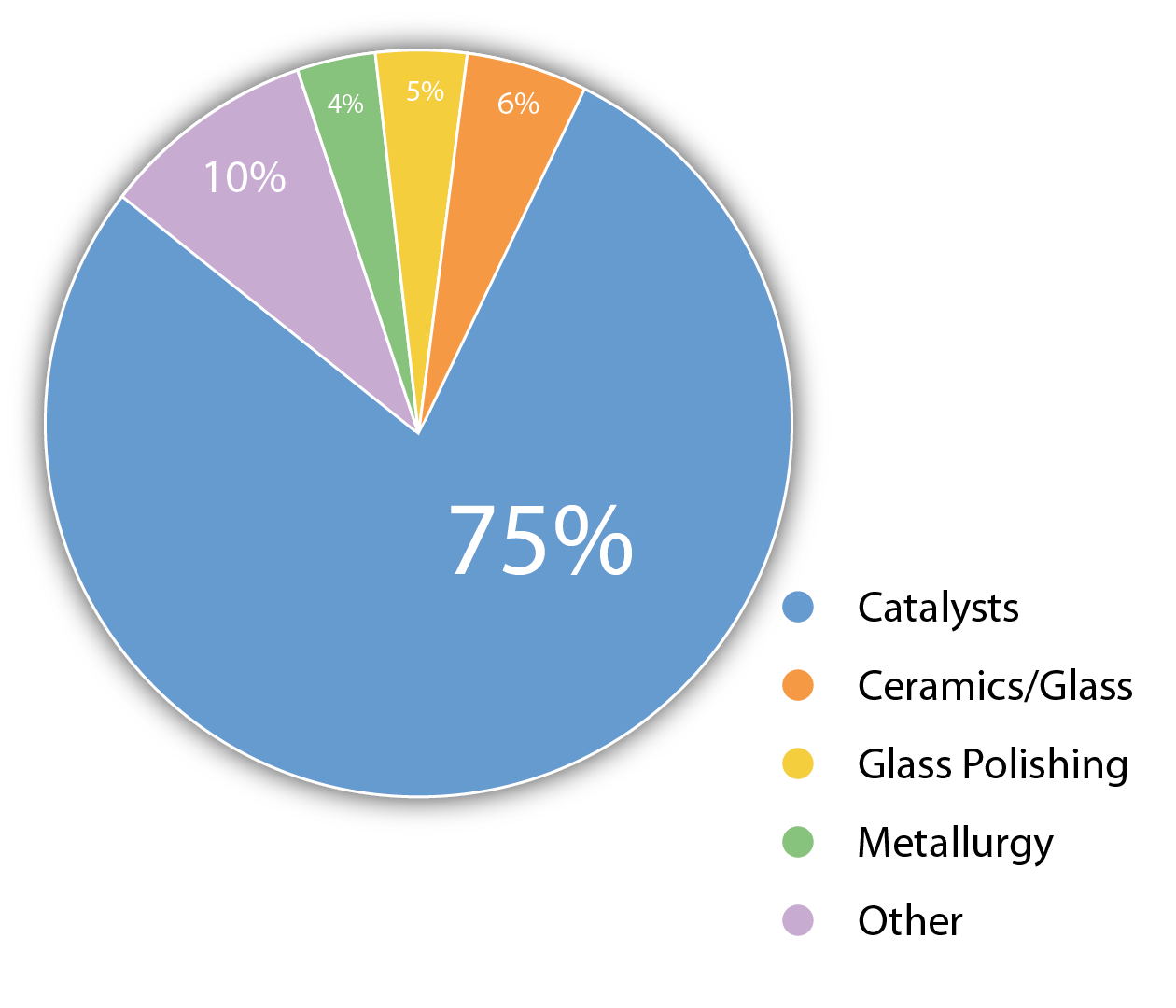
In a variety of contexts, rare earth compounds are valued as catalysts: compounds that facilitate specific chemical reactions without being consumed in the reaction. Catalytic converters in automobiles use rare earth catalysts to react with toxic byproducts of fuel combustion, thereby making vehicle emissions less damaging to the environment. The oil industry uses rare earth-containing catalysts in the process of fluid catalytic cracking (FCC), which converts crude oil to refined products such as gasoline. Finally, in both research and industrial applications, rare earth compounds are used to catalyze a variety of organic synthesis reactions.
Alloys
Rare earth compounds are commonly added to metallic alloys to manipulate key properties of the final material, including grain size, malleability, ductility, resistance to impact, and sensitivity to variable or extreme conditions. Due to the expense of most rare earths, rare earth-containing alloys are frequently designed specifically for particularly demanding applications.
Rare Earth Elements by Applications
Batteries
Nickel-metal hydride (NiMH) batteries are a common type of rechargeable battery, used for both standard AA and AAA batteries for personal electronics and in more demanding applications as batteries for electric vehicles and as backup power sources for telecommunications applications. These batteries are valued for their relatively high energy density, which approaches that of lithium-ion batteries, and for their low temperature sensitivity compared to that of lead-acid batteries. Additionally, NiMH batteries were a desirable replacement for now mostly obsolete nickel-cadmium (NiCd) rechargeable batteries, as none of their components present environmental concerns comparable to the problem of cadmium toxicity. The vast majority of NiMH use a mixture of rare earth metals and more common metals at the negative electrode, as alternate compositions for the metal at this electrode tend to produce batteries with shorter lives.
Fuel Cells

Fuel cells can use rare earth elements in several ways: as catalysts for the fuel cell reaction, as stabilizers of the crystal often used for the electrolyte, and in interlayer materials between cells used to prevent unwanted reactions. Some types of fuel cells use other elements for all of these functions and do not require rare earth compounds at all, while others, such as solid oxide fuel cells (SOFCs), require them for all three functions, though the amounts used still vary considerably based on the individual fuel cell’s design.
Glass and Ceramics

Rare earth elements and compounds are often used as additives in the production of glass and ceramics, or in special coatings for glass surfaces. The rare earths can be used to impart a variety of desirable properties to glasses and ceramics, including increased melting point and shock resistance and low rates of thermal expansion, but are most often noted for their optical properties. Depending on the application, rare earth additives may be used simply as coloring for aesthetic purposes, or may be used to block the transmission of specific wavelengths of light through windows, safety goggles, or optical filters.
Polishing
Cerium(IV) oxide is considered the superior abrasive for polishing of high-quality glass optical components. Additionally, nanostructured cerium oxide is used for the cleaning and polishing of silicon wafers in preparation for their use in integrated circuits or photovoltaics.
Permanent Magnets
Individual atoms of most rare earth elements can retain high magnetic moments in the solid state. In their pure form, these elements only act as permanent magnets at very low temperatures, but they can form compounds with transition metals that act as permanent magnets well above room temperature. These compounds are used in the production of rare earth magnets, which produce significantly stronger magnetic fields than alnico or ferrite magnets. The two types of rare earth magnets currently in use are neodymium magnets, which are comprised of an alloy of neodymium, iron, and boron, and samarium-cobalt magnets, which are an alloy of samarium and cobalt.
The high strength of rare earth magnets means smaller and lighter magnets can replace ferrite/alnico magnets for any given application. Therefore rare earth metals are in high demand in any application where strong permanent magnets are desirable and where size or weight of the magnet is a concern, including in electric motors and generators, hard disk drives, magnetic fasteners, speakers and headphones, and magnetic resonance imaging (MRI) machines. Additionally, rare earth elements can be used to produce magnetic thin films used in digital information storage applications.
Phosphors
Phosphors are compounds that release energy in the form of light when stimulated by a particular energy source. Inorganic phosphors are usually made from a suitable host material—generally oxides, nitrides, sulfides, selenides, halides, or silicates of various metallic elements including some rare earths—with an added activator. The activator is a deliberately introduced impurity that causes deviations in the typical crystal structure called emission centers, which are responsible for the production of light by the phosphor.
Rare earth elements are used as activators in many phosphors. Rare-earth containing phosphors that produce light in response to the absorption energy in the form of light or electricity are a necessity for many everyday technologies, including fluorescent lights, display screens, sensors, and white LEDs. Additionally, there is a special class of phosphors known as scintillators which produce light in response to the absorption of ionizing radiation. Scintillators are essential to the production of radiation detectors and of sensors in medical diagnostic devices such as CT scanners, as well as to certain domains within experimental physics.
Lasers
All lasers produce a beam of electromagnetic radiation by a process of stimulated emission, in which an outside energy source is used to promote electrons within a gain medium to higher energy—“excited”-- quantum states. When these electrons return to their base states, energy is emitted in the form of a photon. Gain mediums are chosen on the basis of many properties, including whether the photons produced are of a desirable wavelength for a given application. Many types of lasers require gain mediums that contain one or more rare earth elements. Notably, crystals used as gain media in solid-state lasers may be doped with neodymium, ytterbium, holmium, thulium, or erbium, and all fiber lasers use rare-earth-doped optical fibers as the gain medium.
Lasers that require rare-earth compounds for construction have a broad range of applications, including material processing (cutting, engraving), telecommunications, spectroscopy, medical diagnosis and treatment, and directed energy weapons.
Medicine
In addition to being components of many key medical technologies, including the magnets in MRIs, sensors in PET machines, phosphor compounds used in some types of medical imaging, and crystals used in lasers for surgery and other treatments, rare earth elements or their compounds can be used more directly in some instances of medical treatment. The compound lanthanum carbonate is used to treat high blood phosphate levels in patients with kidney disease. Additionally, radioactive isotopes of yttrium and lutetium are used for targeted radiation therapy in some cancers, and an isotope of samarium is used to treat pain when cancer spreads to the bone. Finally, yttria-stabilized zirconia is used to fabricate needles for severing individual spinal nerves, as this substance can hold a sharper edge than conventional scalpels.
Below is only a limited selection of the full catalog of Rare Earth products that American Elements manufactures. If you do not see a material you're looking for listed, please search the website or contact customerservice@americanelements.com.
American Elements manufactures a comprehensive catalog of rare earth materials, including high purity and ultra high purity metals, oxides, compounds, and organometallics in forms such as powders, nanopowders, solutions, and sputtering targets. We can custom manufacture all materials to customer specifications for shape, size, purity, composition in all amounts including bulk quantities. Above is a select list of AE Rare Earths products; you may also request a quote directly for any material we produce.