About Antimony
For thousands of years, humans have used antimony in numerous and often radically different ways. As early as 3100 BCE, the mineral stibnite (antimony trisulfide) was used to produce kohl, the jet black eye makeup favored by Ancient Egyptian women, and a vibrant yellow pigment produced from antimony trioxide and lead was used in glassware and paints starting around the 14th century BCE. A potentially apocryphal legend relates how the Babylonian king Nebuchadnezzar was slowly driven mad from exposure to the painted walls of his palace, yet this pigment--which eventually became known as “Naples Yellow”--reached the height of its popularity in the 18th century CE. The writings of first century CE Greek philosopher Pliny the Elder contain a reference to the medicinal uses of stibnite, from which the element lead (having misidentified antimony) could be extracted via heating. The first authors to describe a means of isolating metallic antimony were Italian metallurgist Vannoccio Biringuccio in 1540 CE and Georgius Agricola in 1556; French chemist Nicolas Lémery was the first to study the element and its compounds in depth, publishing his findings in 1707.
The medieval alchemists recognized antimony as a “mundane element” associated with femininity, and gave the element its own symbol (a version of which persists as the symbol for female). Antimony compounds had been used medicinally since the Ancient Greeks prescribed certain powders for the treatment of skin diseases, but they gained popularity as medicinal remedies known as “antimonials” in the years following the death of Swiss-German alchemist and physicist Paracelsus in the 16th century. Paracelsus in particular strongly favored the use of antimonials as purgatives; his therapies were embraced in the subsequent two centuries by many in Europe, particularly in the form of emetics and laxatives, whose notable effectiveness stemmed in large part from their toxicity. Though arsenic is far more deadly, antimony poisoning has similar symptoms, and nearly all forms can have pronounced toxic effects over time, including liver damage or cancer. Elemental antimony is more toxic than its salts, and compounds containing antimony in its trivalent oxidation state are generally ten times more toxic than those containing pentavalent antimony. Stibine (SbH3) and stibnite (Sb2S3) are extremely toxic antimony compounds; exposure to more than 50 mg/m3 they are considered an immediate danger to life and health.
Like other elements including boron, silicon, germanium, arsenic, and tellurium, antimony is classified as a metalloid, having properties somewhere in between those of metals and non-metals. Though its chemical structure is closer to that of true metals, it is less thermally and electrically conductive, and has the unusual property of having a lower electrical conductivity as a solid than a liquid. As is the case for phosphorus and arsenic, several allotropic forms of antimony exist: one stable form, which is a silvery-white metal, and three metastable forms: black, yellow, and explosive. Elemental antimony is resistant to attack by acids and stable in air, though flammable when heated, and it is one of five elements with the unusual property of expanding in volume when solidified (silicon, germanium, gallium, and bismuth being the other four). When molten antimony is allowed to cool, its surface gains a thin crystalline film with a distinctive crystalline fern or star like pattern.
Some niche applications for antimony-based compounds do still exist in the field of medicine. Antimony potassium tartrate or potassium antimony(III) tartrate, better known as tartar emetic, is slowly being phased out, but compounds like meglumine antimonite, sodium stibogluconate, and lithium antimony thiomalate have been used to combat difficult-to-treat parasitic diseases like cutaneous leishmaniasis. However, with the slow recognition of the element’s associated risks came a general shift in its applications towards primarily industrial and high technology uses. Antimony oxides and sodium antimonate are frequently used as flame-retardants in plastics, textiles, leather, and PVC, as exposure to fire causes the release of unstable compounds that quickly combine with oxygen in the air and thus smother the flames. Flame retardants are one of primary industrial uses for antimony. In addition, the heads of safety matches contain a combination of antimony trisulfide and an oxidizing agent such as potassium chlorate with tips of red phosphorus, requiring them to be struck on the specific matchbox surface in order to ignite. Various antimony compounds are used as components of pigments, mordants, pyrotechnics, fining agent to remove gas bubbles in glass, and in the manufacturing of high quality transparent glass used in computer monitors and television screens. In laboratory chemistry, fluoroantimonic acid, obtained from reacting antimony pentafluoride with hydrogen fluoride, is the strongest known superacid.
Recent applications for antimony have focused on advanced semiconductor technologies. Particularly important are the compounds of antimony with indium, gallium, germanium, and tellurium, producing compounds such as InSb, Ge3Sb3, GaSb, and Sb2Te3. These semiconducting compounds are used as components of and as substrates for high-k dielectric materials in laser diodes, integrated circuits, infrared detectors, Hall-effect devices, and memory devices for data storage. Additionally, antimony semiconductors have been proposed to be of critical importance to the development of the next generation of metal-oxide-semiconductor field effect transistors (MOSFETs) and tunnel field effect transistors (TFETs) that could power fast and efficient computers for use in sensors and microelectronics. High purity antimony (99.999+%) serves as an n-type dopant in silicon wafers, and indium tin oxide (ITO) nanoparticles doped with varying concentrations of antimony have been shown to improved laser reflection performance. Several antimony compounds function as topological insulators, hybrid materials that are electrical insulators in their interiors but are conductors at their surface. Silver antimony telluride crystals have been used to develop modeling tools for engineering new thermoelectric devices. and several antimony-based materials like copper antimonide and monodisperse antimony nanocrystals have been investigated as highly efficient electrode and electrolyte materials in next-generation batteries.
Antimony takes its elemental symbol Sb from stibium, the Latin name for stibnite; the origin of the name “antimony” is less clear. Suggestions include the Latin antimonium, which first appeared in a Latin translation of the work by Ceber, the Arabic phrase mesdemet, and the Greek words anti and monos, meaning “not alone,” owing to the fact that the element occurs primarily in compound form or with other heavier metals. Its most common mineral sources are the aforementioned stibnite (also known as antimony-glance), found in hydrothermally formed veins, valentinite (antimony trioxide, a byproduct of the decomposition of stibnite), and tetrahedrite, but the element also occurs in over one hundred different minerals: cervantite or kermesite, senarmontite, nadorite, and more. Antimony is obtained primarily from stibnite during the production of silver, gold, and copper, and can also be recovered during recycling of lead-acid batteries.
Products
 Antimony is available as
Antimony is available as  metal and compounds with purities from 99% to 99.9999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowders. Antimony trioxide is an insoluble form of antimony available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Antimony fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Antimony is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
metal and compounds with purities from 99% to 99.9999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowders. Antimony trioxide is an insoluble form of antimony available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Antimony fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Antimony is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Antimony Properties
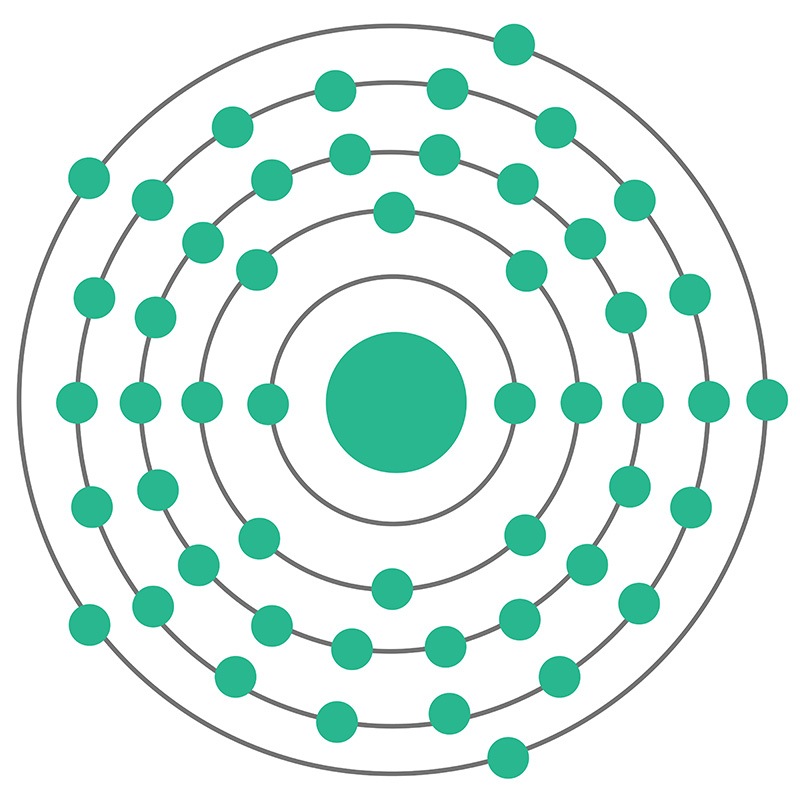
 Antimony is a Block P, Group 15, Period 5 element. The number of electrons in each of Antimony's shells is 2, 8, 18, 18, 5 and its electronic configuration is [Kr] 4d10 5s2 5p3. In its elemental form, CAS 7440-36-0, antimony has a silvery lustrous gray appearance. The antimony atom has a radius of 145.pm and its Van der Waals radius is 200.pm.
Antimony is a Block P, Group 15, Period 5 element. The number of electrons in each of Antimony's shells is 2, 8, 18, 18, 5 and its electronic configuration is [Kr] 4d10 5s2 5p3. In its elemental form, CAS 7440-36-0, antimony has a silvery lustrous gray appearance. The antimony atom has a radius of 145.pm and its Van der Waals radius is 200.pm. 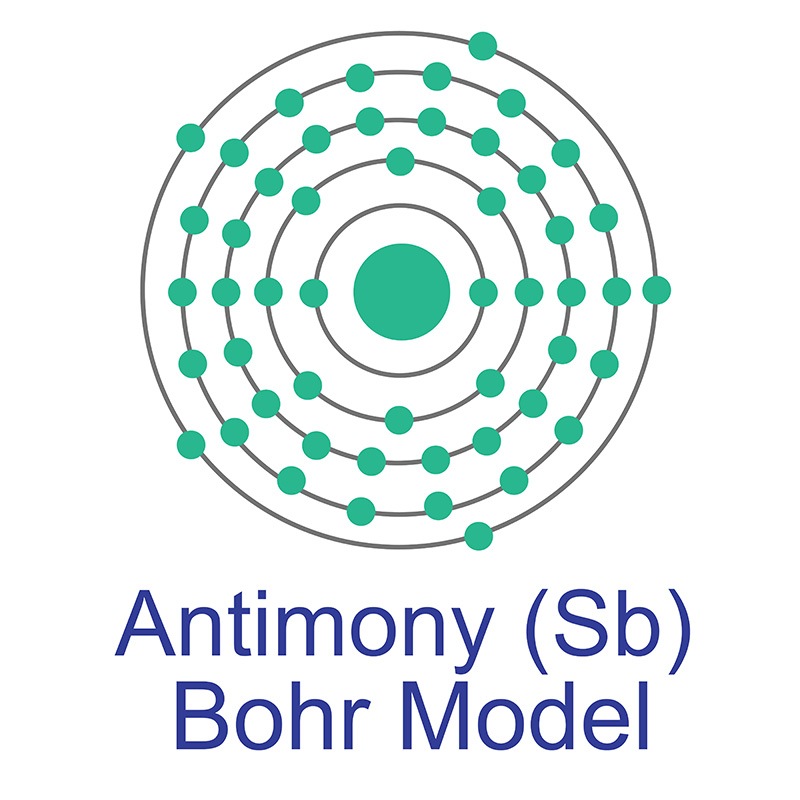 The most common source of Antimony is sulfide stibnite (Sb2S3), although it is sometimes found natively. Antimony is finding use in infrared detectors, diodes and Hall-effect devices as a component of semiconductor compounds such as antimony telluride and gallium antimonide. Antimony itself is, however,
The most common source of Antimony is sulfide stibnite (Sb2S3), although it is sometimes found natively. Antimony is finding use in infrared detectors, diodes and Hall-effect devices as a component of semiconductor compounds such as antimony telluride and gallium antimonide. Antimony itself is, however,  a poor conductor of heat and electricity. The incorporation of a small amount of antimony greatly increases the hardness and mechanical strength of lead, and the resultant alloy has found applications in batteries, antifriction alloys, small arms and tracer bullets and cable sheathing. Antimony compounds are used in manufacturing flame-proofing compounds, paints, ceramic enamels, glass, and pottery glazes. Antimony information, including technical data, safety data, high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
a poor conductor of heat and electricity. The incorporation of a small amount of antimony greatly increases the hardness and mechanical strength of lead, and the resultant alloy has found applications in batteries, antifriction alloys, small arms and tracer bullets and cable sheathing. Antimony compounds are used in manufacturing flame-proofing compounds, paints, ceramic enamels, glass, and pottery glazes. Antimony information, including technical data, safety data, high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
Health, Safety & Transportation Information for Elemental Antimony
The chemical state of antimony affects the toxicity of the element and its compounds. Antimony toxicity makes it immediately dangerous to life or health at 50 mg/m3 or above. Safety data for Antimony metal, nanoparticles and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific antimony material or compound referenced in the “Products” tab. The below information applies to elemental (metallic) Antimony.
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H332-H411 |
| Hazard Codes | N |
| Risk Codes | 51/53 |
| Safety Precautions | 60 |
| RTECS Number | CC4025000 |
| Transport Information | UN 2871 6.1/PG 3 |
| WGK Germany | 2 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Antimony Isotopes
Antimony (Sb) has two stable isotopes and thirty-five radioactive isotopes with half lives ranging from 2.75856 years to less than one hour.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 103Sb | 102.93969(32)# | 100# ms [>1.5 µs] | ß+ to 103Sn | 5/2+# | N/A | 840.1 | - |
| 104Sb | 103.93647(39)# | 0.47(13) s [0.44(+15-11) s] | ß+ to 104Sn; p to 103Sn; ß+ + p to 103In; a to 100In | N/A | N/A | 848.17 | - |
| 105Sb | 104.93149(11) | 1.12(16) s | ß+ to 105Sn; p to 104Sn; ß+ + p to 104In | (5/2+) | N/A | 856.25 | - |
| 106Sb | 105.92879(34)# | 0.6(2) s | ß+ to 106Sn | (4+) | N/A | 873.65 | - |
| 107Sb | 106.92415(32)# | 4.0(2) s | ß+ to 107Sn | 5/2+# | N/A | 881.73 | - |
| 108Sb | 107.92216(22)# | 7.4(3) s | ß+ to 108Sn | (4+) | N/A | 889.81 | - |
| 109Sb | 108.918132(20) | 17.3(5) s | ß+ to 109Sn | 5/2+# | N/A | 907.2 | - |
| 110Sb | 109.91675(22)# | 23.0(4) s | ß+ to 110Sn | (4+) | N/A | 915.28 | - |
| 111Sb | 110.91316(3) | 75(1) s | ß+ to 111Sn | (5/2+) | N/A | 923.36 | - |
| 112Sb | 111.912398(19) | 51.4(10) s | ß+ to 112Sn | 3+ | N/A | 931.44 | - |
| 113Sb | 112.909372(19) | 6.67(7) min | ß+ to 113Sn | 5/2+ | N/A | 948.83 | - |
| 114Sb | 113.90927(3) | 3.49(3) min | ß+ to 114Sn | (3+) | N/A | 956.91 | - |
| 115Sb | 114.906598(17) | 32.1(3) min | ß+ to 115Sn | 5/2+ | N/A | 964.99 | - |
| 116Sb | 115.906794(6) | 15.8(8) min | ß+ to 116Sn | 3+ | N/A | 973.07 | - |
| 117Sb | 116.904836(10) | 2.80(1) h | EC to 117Sn | 5/2+ | N/A | 981.15 | - |
| 118Sb | 117.905529(4) | 3.6(1) min | EC to 118Sn | 1+ | N/A | 989.23 | - |
| 119Sb | 118.903942(9) | 38.19(22) h | EC to 119Sn | 5/2+ | 3.45 | 997.3 | - |
| 120Sb | 119.905072(8) | 15.89(4) min | EC to 120Sn | 1+ | 2.3 | 1005.38 | - |
| 121Sb | 120.9038157(24) | STABLE | - | 5/2+ | 3.3634 | 1013.46 | 57.21 |
| 122Sb | 121.9051737(24) | 2.7238(2) d | EC to 122Sn; ß- to 122Te | 2- | -1.9 | 1021.54 | - |
| 123Sb | 122.9042140(22) | STABLE | - | 7/2+ | 2.5498 | 1029.62 | 42.79 |
| 124Sb | 123.9059357(22) | 60.20(3) d | ß- to 124Te | 3- | 1.2 | 1037.7 | - |
| 125Sb | 124.9052538(28) | 2.75856(25) y | ß- to 125Te | 7/2+ | 2.63 | 1045.78 | - |
| 126Sb | 125.90725(3) | 12.35(6) d | ß- to 126Te | (8-) | 1.3 | 1053.85 | - |
| 127Sb | 126.906924(6) | 3.85(5) d | ß- to 127Te | 7/2+ | 2.7 | 1061.93 | - |
| 128Sb | 127.909169(27) | 9.01(4) h | ß- to 128Te | 8- | 1.3 | 1070.01 | - |
| 129Sb | 128.909148(23) | 4.40(1) h | ß- to 129Te | 7/2+ | N/A | 1078.09 | - |
| 130Sb | 129.911656(18) | 39.5(8) min | ß- to 130Te | (8-)# | N/A | 1076.85 | - |
| 131Sb | 130.911982(22) | 23.03(4) min | ß- to 131Te | (7/2+) | N/A | 1084.93 | - |
| 132Sb | 131.914467(15) | 2.79(5) min | ß- to 132Te | (4+) | N/A | 1093.01 | - |
| 133Sb | 132.915252(27) | 2.5(1) min | ß- to 133Te | (7/2+) | N/A | 1101.09 | - |
| 134Sb | 133.92038(5) | 0.78(6) s | ß- to 134Te | (0-) | N/A | 1099.85 | - |
| 135Sb | 134.92517(11) | 1.68(2) s | ß- to 135Te; ß- + n to 134Te | (7/2+) | N/A | 1107.93 | - |
| 136Sb | 135.93035(32)# | 0.923(14) s | ß- to 136Te; ß- + n to 135Te | 1-# | N/A | 1106.69 | - |
| 137Sb | 136.93531(43)# | 450(50) ms | ß- to 137Te; ß- + n to 136Te | 7/2+# | N/A | 1114.77 | - |
| 138Sb | 137.94079(32)# | 500# ms [>300 ns] | ß- to 138Te; ß- + n to 136Te | 2-# | N/A | 1113.53 | - |
| 139Sb | 138.94598(54)# | 300# ms [>300 ns] | ß- to 139Te | 7/2+# | N/A | 1121.61 | - |