About Boron
Boron is the only non-metallic element of the periodic table family that includes aluminum, gallium, and indium. Classified as a metalloid, or semimetal, the element can mimic the typical behavior of either a metal or a nonmetal, depending on the type of reaction involved. The only nonmetal with 3 electrons in its outer shell to contribute to bonds, boron is a strong electron-pair acceptor (a strong Lewis acid) with a very high affinity for oxygen and is one of the few elements along with carbon and nitrogen known to form stable compounds with triple bonds. Like its neighbor carbon, elemental boron has multiple allotropes, or structural forms, all of which are extremely hard: crystalline boron is a brittle, lustrous sold ranging in appearance from jet black to silvery gray that is a poor electrical conductor; another crystalline form is red in appearance; and amorphous boron is a dark brown powder lower in density than the crystalline forms. Boron is also similar to carbon in that both are able to form covalently bonded networks of molecules, and the two are the only elements that form multiple hydride compounds.
The name boron comes from the Persian or Arabic terms for the mineral borax (burah or buraq, respectively). Borax and boric acid have been known since ancient times in Europe, Tibet, and China, being used by craftsmen to reduce melting point of other materials used in glassmaking and other applications. The first written reference to boron-containing compounds was by the 9th century Persian alchemist Rhazes, who classified one of six groups of minerals as “boraces.” The element was first isolated in 1808 independently by English chemist Sir Humphry Davy and French chemists Joseph-Louis Gay-Lussac and Louis-Jacque Thenard. Later, Henry Moisson isolated a more pure sample by reducing boron trioxide with magnesium, a method that still bears his name.
The chief commercial application for boron is the use of borax (sodium tetraborate decahydrate, Na2B4O7• 10H2O) in soaps and household detergents, water-softening compounds to remove alkaline-earth ions like calcium and magnesium from water, adhesives, cosmetics, fire retardants, and glazes. It is also used to disinfect fruit and lumber, and additionally plays a role in the manufacturing of paper, leather, and plastics. Sodium borate is used to manufacture borosilicate glass, a high-strength glass that is resistant to heat, chemical attack, and thermal shock; applications for borosilicate glasses include bakeware, laboratory containers, and high quality optical glass, as well as serving as precursor materials for glass fiber insulation and textile glass fibers. Boron-containing glasses are generally preferred for flat display panel glass, as in LCDs, as such glasses have superior electrical and strength characteristics as compared to other glasses available for these applications.
Boron is also used in a number of alloy products, including neodymium iron boron (NdFeB) magnets, which are the strongest magnets available and are found in microphones, magnetic switches, speakers, particle accelerators, and dozens of other electronic applications. Boron is also an important dopant molecule used in the production of semiconductor crystals. A number of boron compounds are also important in electronics, including the neutron-detecting scintillator material cadmium borate, and boron subphtalocyanines, organoboron dye molecules with optical properties that are of use in organic LEDs and photovoltaics. Some other organoboron compounds have medical uses, either as treatment agents, or as diagnostic markers.
Though not technically a metal, boron is playing an increasingly important role in advanced materials science due in part to the unique properties of hexagonal boron nitride (hBN), a semiconducting material capable of forming two-dimensional sheets similar to carbon-based graphene. Boron nitride and boron carbide are both superhard materials, and are used widely in cutting tools and as abrasives. Boron carbide is also a strong, heat-resistant material with a high strength-to-weight ratio, making it useful for armor and bulletproof vests. Additionally, boron carbide can absorb neutrons without forming dangerous long-lived radioactive waste, and it is therefore an important material in nuclear power plants. A number of other engineered advanced materials also contain boron. These include borane derivatives, of interest for a variety of specialized applications including hydrogen storage, and lithium borosilicide, a key component of lithium ion batteries.
Boron is relatively scarce element in the universe, produced only via cosmic ray spallation rather than in the cores of stars. Despite composing merely 0.0003% of the earth’s crust by mass, boron is very susceptible to fractionation in the earth’s crust and therefore tends to be concentrated in an deposits of an extensive array of borate (boron oxide)-containing minerals such as borax and kernite (its most common sources), colemanite, and ulexite. It is also present in meteorites, seawater, as a trace element in soils, and silicate minerals like tourmaline and datolite. Methods of obtaining boron include heating the oxide with powdered magnesium or aluminum, or passing an electric current through molten boron trichloride. The city of Boron in California, United States was named after the element and is home to the U.S. Borax Boron Mine, the world’s largest borax mine. The other major source of the element is deposits in Turkey, although the element also is known to accumulate in areas of thermal and volcanic activity.
Products
Boron has an energy band gap of 1.50 to 1.56 eV, which is higher than that of either silicon or germanium. Optical characteristics include transmitting portions of the infrared. Boron is a poor conductor of electricity at room temperature but a good conductor at high temperature. Amorphous boron is used in pyrotechnic flares to provide a distinctive green color, and in rockets as an igniter. Boric acid is an important boron compound with major markets in textile products. Boron compounds 
 are used extensively in the manufacture of borosilicate glasses. The isotope Boron-10 is used as a control for nuclear reactors, as a shield for nuclear radiation, and in instruments used for detecting neutrons. Boron nitride has remarkable properties and can be used to make a material as hard as diamond. The nitride also behaves like an electrical insulator but conducts heat like a metal. Boron is available in forms with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental forms include pellets, rod, wire and granules for evaporation source material purposes. Boron oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Boron fluoride is an insoluble source of fluoride for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Boron is also available in soluble forms including chlorides, and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
are used extensively in the manufacture of borosilicate glasses. The isotope Boron-10 is used as a control for nuclear reactors, as a shield for nuclear radiation, and in instruments used for detecting neutrons. Boron nitride has remarkable properties and can be used to make a material as hard as diamond. The nitride also behaves like an electrical insulator but conducts heat like a metal. Boron is available in forms with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental forms include pellets, rod, wire and granules for evaporation source material purposes. Boron oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Boron fluoride is an insoluble source of fluoride for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Boron is also available in soluble forms including chlorides, and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Boron Properties
 Boron is a Block P, Group 13, Period 2 element.
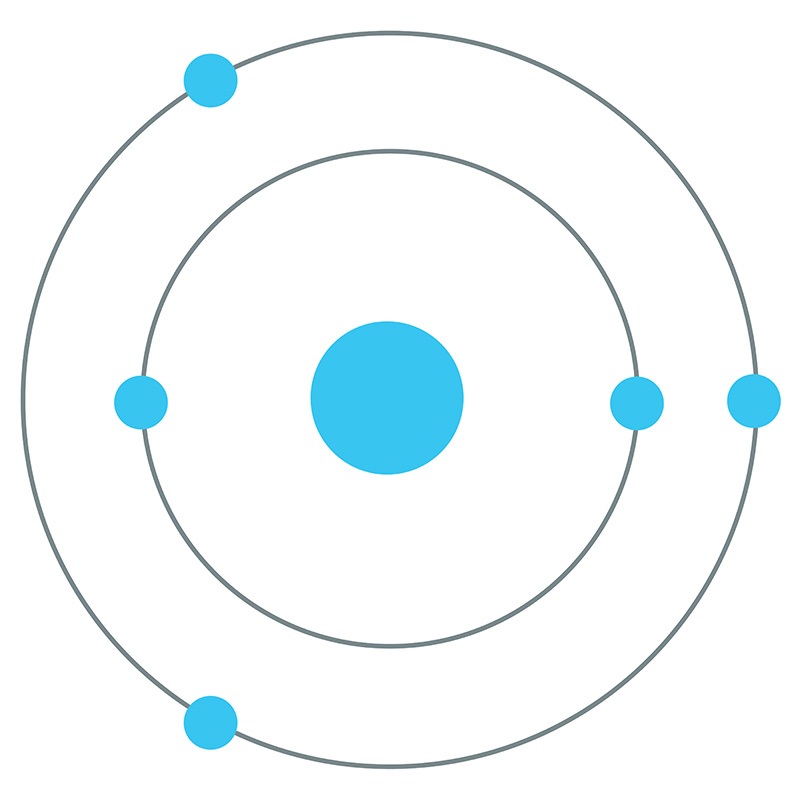
Boron is a Block P, Group 13, Period 2 element.  The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90.pm and its Van der Waals radius is 192.pm.
The number of electrons in each of boron's shells is 2, 3 and its electron configuration is [He] 2s2 2p1. The boron atom has a radius of 90.pm and its Van der Waals radius is 192.pm.  In its elemental form, CAS 7440-42-8, boron has a black-brown appearance. Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable compounds featuring triple bonds. Boron is found in borates, borax, boric acid, colemanite, kernite, and ulexite. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808. It was first isolated by Humphry Davy, also in 1808. The name Boron originates from a combination of carbon and the Arabic word buraqu meaning borax.
In its elemental form, CAS 7440-42-8, boron has a black-brown appearance. Along with carbon and nitrogen, boron is one of the few elements in the periodic table known to form stable compounds featuring triple bonds. Boron is found in borates, borax, boric acid, colemanite, kernite, and ulexite. Boron was discovered by Joseph Louis Gay-Lussac and Louis Jacques Thénard in 1808. It was first isolated by Humphry Davy, also in 1808. The name Boron originates from a combination of carbon and the Arabic word buraqu meaning borax.
Health, Safety & Transportation Information for Boron
Boron in its elemental form is not toxic. Safety data for Boron and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental Boron.
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Codes | 22 |
| Safety Precautions | N/A |
| RTECS Number | ED7350000 |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Boron Isotopes
Boron has two stable isotopes: 10B and 11B.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 6B | 6.04681(75)# | N/A | Unknown | N/A | N/A | -0.74 | - |
| 7B | 7.02992(8) | 350(50)E-24 s [1.4(2) ] | p to 6Be | (3/2-) | N/A | 23.08 | - |
| 8B | 8.0246072(11) | 770(3) ms | EC to 8Be; 4He; EC + 2 a to n | 2+ | 1.0355 | 36.1 | - |
| 9B | 9.0133288(11) | 800(300)E-21 s [0.54(21) keV] | 2a to 1H; p to 8Be | 3/2- | 1.8006 | 54.71 | - |
| 10B | 10.0129370(4) | STABLE | - | 3+ | 1.80065 | 64 | 19.9 |
| 11B | 11.0093054(4) | STABLE | - | 3/2- | 2.688637 | 74.87 | 80.1 |
| 12B | 12.0143521(15) | 20.20(2) ms | ß- to 12C; ß- + 3a to n | 1+ | 1.0031 | 78.29 | - |
| 13B | 13.0177802(12) | 17.33(17) ms | ß- to 13C; ß- + n to 12C | 3/2- | 3.17778 | 83.58 | - |
| 14B | 14.025404(23) | 12.5(5) ms | ß- to 14C; ß- + n to 13C | 2- | N/A | 84.2 | - |
| 15B | 15.031103(24) | 9.87(7) ms | ß- + n to 14C; ß- to 15C; ß- + 2n to 13C | 3/2- | N/A | 86.69 | - |
| 16B | 16.03981(6) | <190E-12 s [<0.1 ] | n to 15B | 0- | N/A | 87.32 | - |
| 17B | 17.04699(18) | 5.08(5) ms | ß- + n to 16C; ß- to 17C; ß- + 2n to 15C; ß- + 3n to 14C; ß- + 4n to 13C | (3/2-) | N/A | 88.87 | - |
| 18B | 18.05617(86)# | >26 ns | n to 17B | (4-)# | N/A | 87.64 | - |
| 19B | 19.06373(43)# | 2.92(13) ms | ß- to 19C | (3/2-)# | N/A | 89.19 | - |