About Indium
In 1863, chemists Ferdinand Reich and Hieronymous Theodor Richter were spectroscopically inspecting various ores in hope of finding the element thallium. Thallium’s characteristic green emission lines were absent, but they did note instead a bright blue spectral line. No element was known that produced such a line, so they correctly assumed that their samples contained a new element. They named the hypothetical new element indium from the indigo of its spectral line, and Richter went on to isolate pure indium metal the following year.
Indium took an exceedingly slow path from initial discovery to commercial relevance. For the first seventy years after its discovery, it was primarily a curiosity. A sample of the metal was presented at the World Fair in 1867, but no major indium mining operations existed until the late 1920’s. These operations were initiated only because several chemists became interested in using indium as a hardening surface treatment for ferrous metals and found that, in order to experiment with this idea, they had to develop a source of the metal themselves. The first large-scale application for the metal was as a coating for bearings in high-performance aircraft engines, but this and several niche alloys were the only sources of demand for the metal until it began to be used in semiconductor technologies starting in 1952. After this point, production gradually increased as applications for the metal both in alloys and in compound semiconductors continued to be developed.
Since 1992, a single semiconducting compound has accounted for the majority of demand for indium: indium tin oxide (ITO). When applied in a thin film to a surface, ITO produces an optically transparent conductive coating, allowing it to serve as a transparent electrode in electronic devices. Transparent electrodes are essential to the design of liquid crystal displays (LCDs), plasma screens, and touchscreen devices. ITO is also used to coat aircraft windshields, which can then be conveniently defrosted when a current is applied across the conducting film, and additionally is found in organic LEDs, solar cells, sodium vapor lamps, antistatic coatings, thin-film strain gauges, and electromagnetic interference shielding.
Though not used at the high volume of ITO, several other indium-based semiconductors have important applications. Most indium semiconductors, including indium arsenide, indium phosphide, indium nitride, indium antimonide, and many alloys of these with other semiconducting compounds, are notable for their high electron mobility, and are therefore found in high-frequency electronics such as high-frequency transistors. Additionally, these materials generally have direct bandgaps, making them suitable for optoelectronic devices such as LEDs, lasers, thin-film solar cells, radiation detectors, and integrated optical circuits. The compound semiconductor copper indium gallium selenide (CIGS) is one of only a few materials currently used commercially for the production of thin-film photovoltaic devices. Additionally, many indium semiconductors are being investigated for potential use in novel nanoengineered forms such as quantum dots and nanowires.
Outside of use in semiconductors, indium is found primarily as a metal, either alone or in alloys, usually in applications that exploit its low melting point. Indium can be used to produce alloys such as Galinstan, which are liquid at room temperature and can replace mercury in applications such as thermometers. Indium alloys are used frequently in seals found in low-temperature applications, as they maintain malleability and ductility at low temperatures. Indium-containing solders are have become more important thanks to increasingly stringent restrictions on the use of lead, another low-melting-point metal once found in most low-temperature solders. Additionally, indium may be found in die casting alloys and in thermal interface materials.
Two niche uses of indium are also notable. A radioisotope of indium is used in indium leukocyte imaging, which is used to track white blood cells in the body in order to identify sites of infection. Indium is also a component of control rods used in nuclear reactors, where it absorbs excess neutrons along with silver and cadmium .
Indium is not particularly rare--it is approximately as abundant as mercury--but there are no economically significant indium ore minerals. The metal must therefore be extracted from other metal ores where it occurs in trace amounts, and today is most commonly extracted from byproducts of zinc mining. Increasingly, indium is recovered from waste material produced by the ITO sputtering process, and even directly from scrap LCD panels. These recycling efforts vary in economic feasibility depending on the efficiency of the process and current prices of the metal, and therefore their use varies widely between countries and from year to year. Concerns about depleting world indium resources have led to substantial interest in developing alternative transparent electrode materials to substitute for ITO in electronics.
Products
 Indium is available as metal and compounds with purities from 99% to 99.9999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
Indium is available as metal and compounds with purities from 99% to 99.9999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.  Indium is also used in various metal alloys (See AE Alloys). Elemental or metallic forms include pellets, wire and granules for evaporation source material purposes. Indium oxide is available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Indium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Indium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Indium is also used in various metal alloys (See AE Alloys). Elemental or metallic forms include pellets, wire and granules for evaporation source material purposes. Indium oxide is available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Indium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Indium is available in soluble forms including chlorides, nitrates and acetates. These compounds are also manufactured as solutions at specified stoichiometries.
Indium Properties
![]() Indium is a Block P, Group 13, Period 5 element.
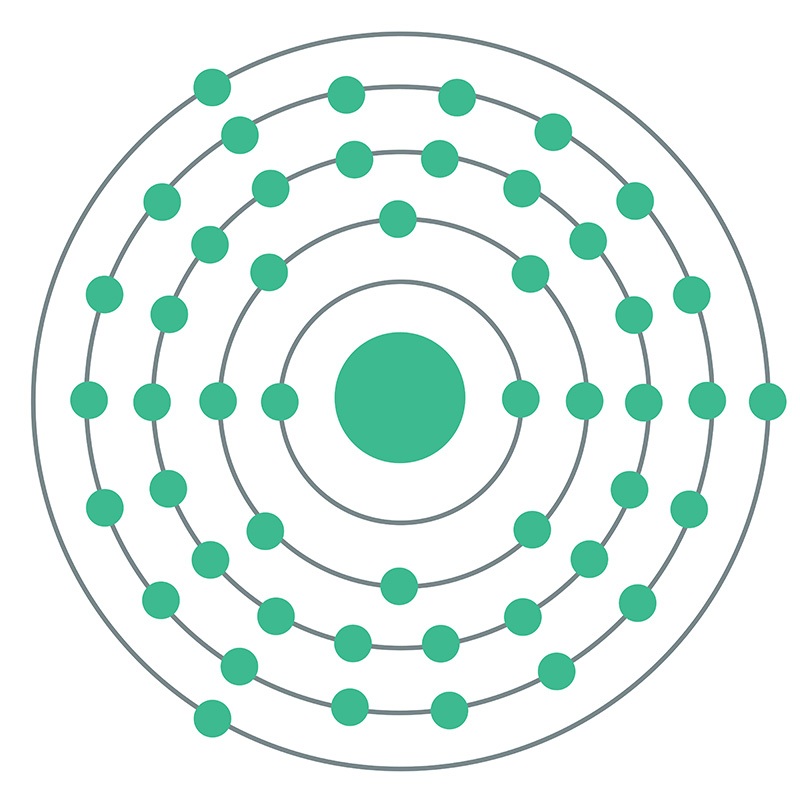
Indium is a Block P, Group 13, Period 5 element. 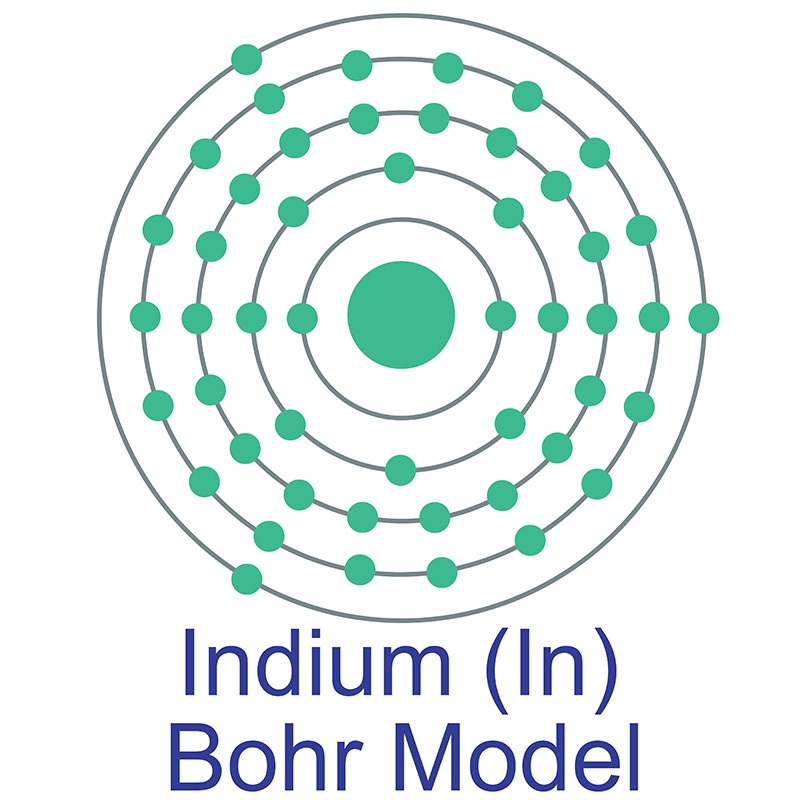 The number of electrons in each of Indium's shells is 2, 8, 18, 18, 3 and its electronic configuration is [Kr] 4d10 5s2 5p1. In its elemental form, CAS 7440-74-6, Indium has a silvery lustrous gray appearance. The indium atom has a radius of 162.6.pm and its Van der Waals radius is 193.pm. Indium has found application in semiconductor devices and other electronic applications. It is used in making bearing alloys, germanium transistors, rectifiers, and photoconductors.
The number of electrons in each of Indium's shells is 2, 8, 18, 18, 3 and its electronic configuration is [Kr] 4d10 5s2 5p1. In its elemental form, CAS 7440-74-6, Indium has a silvery lustrous gray appearance. The indium atom has a radius of 162.6.pm and its Van der Waals radius is 193.pm. Indium has found application in semiconductor devices and other electronic applications. It is used in making bearing alloys, germanium transistors, rectifiers, and photoconductors.  Indium is used to make low-melting alloys. Indium can be plated onto metal and evaporated onto glass, forming a mirror that performs as well as those made with silver and which better resists atmospheric corrosion. Indium information, including technical data, safety data, high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
Indium is used to make low-melting alloys. Indium can be plated onto metal and evaporated onto glass, forming a mirror that performs as well as those made with silver and which better resists atmospheric corrosion. Indium information, including technical data, safety data, high purity properties, research, applications and other useful facts are discussed below. Scientific facts such as the atomic structure, ionization energy, abundance on earth, conductivity and thermal properties are also included.
Health, Safety & Transportation Information for Indium
Indium is only slightly toxic. Safety data for Indium metal, nanoparticles and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific indium material or compound referenced in the Products tab. The below information applies to elemental (metallic) Indium.
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H315-H319-H332-H335 |
| Hazard Codes | Xn |
| Risk Codes | 20/21/22-36/37/38 |
| Safety Precautions | 26-36 |
| RTECS Number | NL1050000 |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Indium Isotopes
Indium has two naturally occuring isotopes: 113In and 115In. The stable isotope, 113In, accounts for only 4.3% of naturally occurring indium. 115In has a half life of 441 trillion years and accounts for 95.7% of naturally occurring indium.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 97In | 96.94954(64)# | 5# ms | Unknown | 9/2+# | N/A | 776.17 | - |
| 98In | 97.94214(21)# | 45(23) ms [32(+32-11) ms] | ß+ to 98Cd | 0+# | N/A | 790.77 | - |
| 99In | 98.93422(43)# | 3.1(8) s [3.0(+8-7) s] | ß+ to 99Cd | 9/2+# | N/A | 806.3 | - |
| 100In | 99.93111(27) | 5.9(2) s | ß+ to 100Cd; ß+ + p to 99Ag | (6,7)+ | N/A | 817.17 | - |
| 101In | 100.92634(32)# | 15.1(3) s | ß+ to 101Cd; ß+ + p to 100Ag | 9/2+# | N/A | 835.5 | - |
| 102In | 101.92409(12) | 23.3(1) s | ß+ to 102Cd; ß+ + p to 101Ag | (6+) | N/A | 843.58 | - |
| 103In | 102.919914(27) | 60(1) s | ß+ to 103Cd | 9/2+# | N/A | 860.97 | - |
| 104In | 103.91830(9) | 1.80(3) min | ß+ to 104Cd | 5,6(+) | N/A | 869.05 | - |
| 105In | 104.914674(19) | 5.07(7) min | ß+ to 105Cd | 9/2+ | N/A | 877.13 | - |
| 106In | 105.913465(13) | 6.2(1) min | ß+ to 106Cd | 7+ | N/A | 885.21 | - |
| 107In | 106.910295(12) | 32.4(3) min | ß+ to 107Cd | 9/2+ | N/A | 893.29 | - |
| 108In | 107.909698(10) | 58.0(12) min | ß+ to 108Cd | 7+ | N/A | 910.68 | - |
| 109In | 108.907151(6) | 4.2(1) h | EC to 109Cd | 9/2+ | 5.54 | 918.76 | - |
| 110In | 109.907165(13) | 4.9(1) h | EC to 110Cd | 7+ | 4.37 | 926.84 | - |
| 111In | 110.905103(5) | 2.8047(5) d | EC to 111Cd | 9/2+ | 5.5 | 934.92 | - |
| 112In | 111.905532(6) | 14.97(10) min | EC to 112Cd; ß- to 112Sn | 1+ | 2.82 | 943 | - |
| 113In | 112.904058(3) | STABLE | - | 9/2+ | 5.5289 | 951.08 | 4.29 |
| 114In | 113.904914(3) | 71.9(1) s | EC to 114Cd; ß- to 114Sn | 1+ | 2.82 | 959.15 | - |
| 115In | 114.903878(5) | 4.41(25)E+14 y | ß- to 115Sn | 9/2+ | 5.5408 | 967.23 | 95.71 |
| 116In | 115.905260(5) | 14.10(3) s | ß- to 116Sn; EC to 116Cd | 1+ | N/A | 975.31 | - |
| 117In | 116.904514(6) | 43.2(3) min | ß- to 117Sn | 9/2+ | N/A | 983.39 | - |
| 118In | 117.906354(9) | 5.0(5) s | ß- to 118Sn | 1+ | N/A | 991.47 | - |
| 119In | 118.905845(8) | 2.4(1) min | ß- to 119Sn | 9/2+ | N/A | 999.55 | - |
| 120In | 119.90796(4) | 3.08(8) s | ß- to 120Sn | 1+ | N/A | 1007.63 | - |
| 121In | 120.907846(29) | 23.1(6) s | ß- to 121Sn | 9/2+ | N/A | 1015.71 | - |
| 122In | 121.91028(5) | 1.5(3) s | ß- to 122Sn | 1+ | N/A | 1014.47 | - |
| 123In | 122.910438(26) | 6.17(5) s | ß- to 123Sn | (9/2)+ | N/A | 1022.55 | - |
| 124In | 123.91318(5) | 3.11(10) s | ß- to 124Sn | 3+ | N/A | 1030.63 | - |
| 125In | 124.91360(3) | 2.36(4) s | ß- to 125Sn | 9/2+ | N/A | 1038.7 | - |
| 126In | 125.91646(4) | 1.53(1) s | ß- to 126Sn | 3(+#) | N/A | 1046.78 | - |
| 127In | 126.91735(4) | 1.09(1) s | ß- to 127Sn; ß- + n to 126Sn | 9/2(+) | N/A | 1054.86 | - |
| 128In | 127.92017(5) | 0.84(6) s | ß- to 128Sn; ß- + n to 127Sn | (3)+ | N/A | 1053.62 | - |
| 129In | 128.92170(5) | 611(4) ms | ß- to 129Sn; ß- + n to 128Sn | 9/2+# | N/A | 1061.7 | - |
| 130In | 129.92497(4) | 0.29(2) s | ß- to 130Sn; ß- + n to 129Sn | 1(-) | N/A | 1069.78 | - |
| 131In | 130.92685(3) | 0.28(3) s | ß- to 131Sn; ß- + n to 130Sn | (9/2+) | N/A | 1077.86 | - |
| 132In | 131.93299(7) | 206(4) ms | ß- to 132Sn; ß- + n to 131Sn | (7-) | N/A | 1076.62 | - |
| 133In | 132.93781(32)# | 165(3) ms | ß- + n to 132Sn; ß- to 133Sn | (9/2+) | N/A | 1084.7 | - |
| 134In | 133.94415(43)# | 140(4) ms | ß- to 134Sn; ß- + n to 133Sn; ß- + 2n to 132Sn | N/A | N/A | 1083.46 | - |
| 135In | 134.94933(54)# | 92(10) ms | Unknown | 9/2+# | N/A | 1091.54 | - |