About Cadmium
In nature, cadmium is most commonly found as a minor component of zinc ores. This fact led to its discovery in 1817 by both Karl Samuel Leberecht Hermann and Friedrich Stromeyer, who was examining impurities in a sample of the zinc carbonate mineral calamine (or cadmia in Latin), from which the name of the element was subsequently derived.
Cadmium found a variety of applications soon after its discovery. The first notable use of cadmium was in red, orange, and yellow pigments based on cadmium sulfides and selenides, which started on a small scale as early as the 1840’s. Cadmium pigments are valued for their vividness and permanence, and at the time of their introduction there were few options for stable pigments in this color range. As industrial scale production of cadmium metal started in the earlier twentieth century, cadmium pigments gained in popularity and other applications followed. Since cadmium is resistant to corrosion, it can be deposited by an electroplating process to serve as a protective coating on more easily corroded metals such as steel. Nickel-cadmium (NiCd) batteries were first invented in 1899, but became commonly produced starting in the mid-1940’s. For the next fifty years, they were the primary rechargeable batteries available for consumer electronics. Cadmium can also be a component of silver-based solder alloys that are versatile and have high strength along with a uniquely low melting point, and cadmium compounds can be used to stabilize PVC plastics, greatly increasing their resistance to heat and general wear.
Unfortunately, using cadmium for all of these applications had one major downside: cadmium and many of its compounds are extremely toxic. Poisoning by the metal’s fumes or cadmium-laden dust is often acute, producing severe flu-like symptoms, respiratory problems, and damage to the liver and kidneys within hours of exposure. Acute organ damage can also result from ingestion of large amounts of cadmium compounds, but long term low-level exposure can also lead to insidious damage, producing progressive kidney disease, gout, and dangerously weak bones which lead to severe pain and fractures. Cadmium in industrial waste, landfills, and mines easily leaches into groundwater, and from there can be consumed in drinking water or accumulate in crops. All plants can absorb some cadmium from the soil, but some are particularly prone to concentrating the metal, which sometimes leads to tragic mass poisonings.
Cadmium's toxicity concerns have led to workplace safety regulations, battery recycling programs, and a substantial decline in traditional uses of the metal. Alternative pigments such as cerium sulfide and azo pigments are now available for many applications, though some fine artist's paints still include cadmium. In most applications of corrosion-resistant thin films, zinc or aluminum plating can serve the same purpose as cadmium. Few solder formulas still include cadmium, and alternative stabilizers have been developed for the manufacture of PVC products. Finally, nickel metal hydride(NiMH) and lithium ion batteries are now becoming economically viable and functionally comparable alternatives to Ni-Cd for rechargeable batteries in consumer electronics, though Ni-Cd batteries still have advantages to recommend them for some specialized applications.
A relatively new application for cadmium in compound semiconductors is becoming increasingly relevant. Cadmium can form II-VI class semiconducting compounds with selenium, tellurium, and sulfur, and can also be a component of several ternary semiconductors. The largest current use of cadmium semiconductors is in cadmium telluride thin-film photovoltaics, but they are also used in radiation detectors, electro-optic modulators, optical windows and lenses, photoresistors, and lasers. Additionally, ongoing research into nanoscale cadmium semiconductor crystals such as cadmium selenide quantum dots has shown promise for a variety of applications, including higher-efficiency LED-type lighting.
Cadmium is relatively rare and there are no common cadmium ores, so today the element is still obtained commercially as a byproduct of zinc mining. Cadmium sulfide is the compound most commonly found in zinc ores, and as it is easy to isolate and purify, it is the primary source of cadmium for industrial applications.
Products
Cadmium is a key component in battery production, certain pigments and coatings, and is commonly used in electroplating. Cadmium oxide is used in phosphors for television picture tubes. Cadmium sulfide (CdS) is used as a photoconductive surface coating for photocopier drums. Cadmium is also used  to absorb neutrons in nuclear reactors. Cadmium in glass and ceramic glazes create a distinctive cadmium yellow.
to absorb neutrons in nuclear reactors. Cadmium in glass and ceramic glazes create a distinctive cadmium yellow.  Cadmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Cadmium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cadmium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Cadmium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Cadmium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Cadmium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Cadmium Properties
 Cadmium is a Block D, Group 12, Period 5 element.
Cadmium is a Block D, Group 12, Period 5 element. 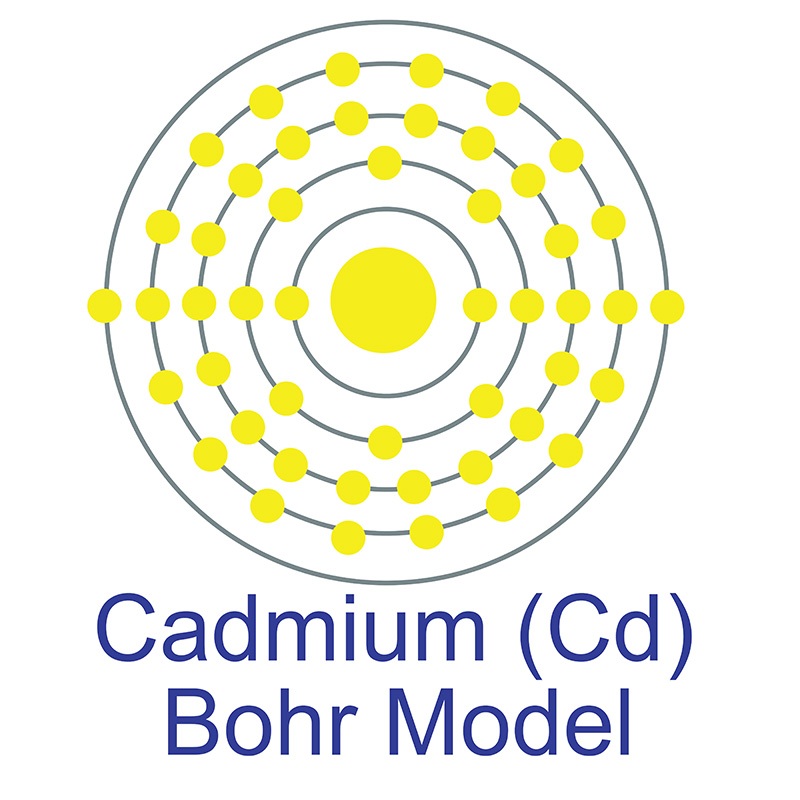 The number of electrons in each of Cadmium's shells is 2, 8, 18, 18, 2 and its electronic configuration is [Kr] 4d10 5s2.
The number of electrons in each of Cadmium's shells is 2, 8, 18, 18, 2 and its electronic configuration is [Kr] 4d10 5s2.  The cadmium atom has a radius of 148.9.pm and its Van der Waals radius is 158.pm. In its elemental form, CAS 7440-43-9, cadmium has a silvery bluish gray metallic appearance. Cadmium makes up about 0.1 ppm of the earth's crust. No significant deposits of cadmium containing ores are known, however, it is sometimes found in its metallic form. It is a common impurity in zinc ores and is isolated during the production of zinc. Cadmium was first discovered by Friedrich Stromeyer in 1817. The name cadmium originates from the Latin word 'cadmia' and the Greek word 'kadmeia'.
The cadmium atom has a radius of 148.9.pm and its Van der Waals radius is 158.pm. In its elemental form, CAS 7440-43-9, cadmium has a silvery bluish gray metallic appearance. Cadmium makes up about 0.1 ppm of the earth's crust. No significant deposits of cadmium containing ores are known, however, it is sometimes found in its metallic form. It is a common impurity in zinc ores and is isolated during the production of zinc. Cadmium was first discovered by Friedrich Stromeyer in 1817. The name cadmium originates from the Latin word 'cadmia' and the Greek word 'kadmeia'.
Health, Safety & Transportation Information for Cadmium
Cadmium and its compounds are toxic. Safety data for Cadmium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Cadmium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H330-H341-H350-H361fd-H372-H410 |
| Hazard Codes | T+,N |
| Risk Codes | 45-26-48/23/25-50/53-62-63-68 |
| Safety Precautions | 53-45-60-61 |
| RTECS Number | EU9800000 |
| Transport Information | UN 2570 6.1/PG 1 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Cadmium Isotopes
Naturally occurring cadmium (Cd) has six stable isotopes: 106Cd, 108Cd, 110Cd, 111Cd, 112Cd, and 114Cd.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 95Cd | 94.94987(64)# | 5# ms | Unknown | 9/2+# | N/A | 761.13 | - |
| 96Cd | 95.93977(54)# | 1# s | ß+ to 96Ag | 0+ | N/A | 778.53 | - |
| 97Cd | 96.93494(43)# | 2.8(6) s | ß+ to 97Ag; ß+ + p to 96Ag | 9/2+# | N/A | 791.26 | - |
| 98Cd | 97.92740(8) | 9.2(3) s | ß+ to 98Ag; ß+ + p to 97Ag | 0+ | N/A | 805.86 | - |
| 99Cd | 98.92501(22)# | 16(3) s | ß+ to 99Ag; ß+ + p to 98Ag; ß+ + a to 98Ag | (5/2+) | N/A | 815.81 | - |
| 100Cd | 99.92029(10) | 49.1(5) s | ß+ to 100Ag | 0+ | N/A | 828.54 | - |
| 101Cd | 100.91868(16) | 1.36(5) min | ß+ to 101Ag | (5/2+) | N/A | 845.94 | - |
| 102Cd | 101.91446(3) | 5.5(5) min | ß+ to 102Ag | 0+ | N/A | 854.02 | - |
| 103Cd | 102.913419(17) | 7.3(1) min | ß+ to 103Ag | 5/2+ | N/A | 862.09 | - |
| 104Cd | 103.909849(10) | 57.7(10) min | ß+ to 104Ag | 0+ | N/A | 879.49 | - |
| 105Cd | 104.909468(12) | 55.5(4) min | ß+ to 105Ag | 5/2+ | N/A | 887.57 | - |
| 106Cd | 105.906459(6) | Observationally Stable | - | 0+ | N/A | 895.65 | 1.25 |
| 107Cd | 106.906618(6) | 6.50(2) h | EC to 107Ag | 5/2+ | -0.615055 | 903.73 | - |
| 108Cd | 107.904184(6) | Observationally Stable | - | 0+ | N/A | 911.8 | 0.89 |
| 109Cd | 108.904982(4) | 461.4(12) d | EC to 109Ag | 5/2+ | -0.827846 | 919.88 | - |
| 110Cd | 109.9030021(29) | STABLE | - | 0+ | N/A | 927.96 | 12.49 |
| 111Cd | 110.9041781(29) | STABLE | - | 1/2+ | -0.5948857 | 936.04 | 12.8 |
| 112Cd | 111.9027578(29) | STABLE | - | 0+ | N/A | 944.12 | 24.13 |
| 113Cd | 112.9044017(29) | 7.7(3)E+15 y | ß- to 113In | 1/2+ | -0.6223005 | 952.2 | 12.22 |
| 114Cd | 113.9033585(29) | Observationally Stable | - | 0+ | N/A | 960.28 | 28.73 |
| 115Cd | 114.9054310(29) | 53.46(5) h | ß- to 115Cd | 1/2+ | -1.087 | 968.36 | - |
| 116Cd | 115.904756(3) | 3.1(4)E+19 y | ß- to 116Sn | 0+ | N/A | 976.43 | 7.49 |
| 117Cd | 116.907219(4) | 2.49(4) h | ß- to 117Cd | 1/2+ | N/A | 984.51 | - |
| 118Cd | 117.906915(22) | 50.3(2) min | ß- to 118Cd | 0+ | N/A | 992.59 | - |
| 119Cd | 118.90992(9) | 2.69(2) min | ß- to 119Cd | (3/2+) | N/A | 1000.67 | - |
| 120Cd | 119.90985(2) | 50.80(21) s | ß- to 120Cd | 0+ | N/A | 1008.75 | - |
| 121Cd | 120.91298(9) | 13.5(3) s | ß- to 121Cd | (3/2+) | N/A | 1007.51 | - |
| 122Cd | 121.91333(5) | 5.24(3) s | ß- to 122Cd | 0+ | N/A | 1015.59 | - |
| 123Cd | 122.91700(4) | 2.10(2) s | ß- to 123Cd | (3/2)+ | N/A | 1023.67 | - |
| 124Cd | 123.91765(7) | 1.25(2) s | ß- to 124Cd | 0+ | N/A | 1031.75 | - |
| 125Cd | 124.92125(7) | 0.65(2) s | ß- to 125Cd | (3/2+)# | N/A | 1030.51 | - |
| 126Cd | 125.92235(6) | 0.515(17) s | ß- to 126Cd | 0+ | N/A | 1038.59 | - |
| 127Cd | 126.92644(8) | 0.37(7) s | ß- to 127Cd | (3/2+) | N/A | 1046.67 | - |
| 128Cd | 127.92776(32) | 0.28(4) s | ß- to 128Cd | 0+ | N/A | 1054.75 | - |
| 129Cd | 128.93215(32)# | 242(8) ms | ß- to 129Cd; IT | 3/2+# | N/A | 1053.51 | - |
| 130Cd | 129.9339(3) | 162(7) ms | ß- to 130Cd; ß- + n to 129Cd; | 0+ | N/A | 1061.59 | - |
| 131Cd | 130.94067(32)# | 68(3) ms | Unknown | 7/2-# | N/A | 1060.35 | - |
| 132Cd | 131.94555(54)# | 97(10) ms | Unknown | 0+ | N/A | 1068.43 | - |