About Lanthanum
Swedish chemist Carl Gustaf Mosander discovered lanthanum during experiments on an impure sample of cerium nitrate in 1839.
Like most of the rare-earth elements, lanthanum is used in small amounts to alter key properties of other materials. Lanthanum oxide is added to glass to impart increased resistance to alkalis, strength, a high refractive index and low dispersion, and can be used to make infrared-absorbing glass. Lanthanum is also an essential component of ZBLAN glass, which exhibits superior transmittance of infrared light and is used in fiber-optic communication systems. These glasses are used in specialized optical applications such as telescope lenses. Lanthanum-doped ceramic materials are used as both anodes and cathodes in solid oxide fuel cells. Adding small quantities of lanthanum to steel improves its malleability, ductility, and resistance to impact, while the addition of lanthanum to molybdenum decreases its hardness and sensitivity to variations in temperature.
Many rare earth compounds have the ability to produce light in response to absorbing energy from an external source. The first commercial use of lanthanum exploited this property for the production of gas lantern mantles, but unfortunately those mantles produced green-tinged light and were not very successful. Their creator Carl Auer von Welsbach found more success with a cerium-containing mantle several years later. Lanthanum is also a component of mischmetal, an alloy used for lighter flints. Today, lanthanum phosphors like lanthanum fluoride are used in fluorescent lamps, and cerium-doped lanthanum bromide and chloride scintillators serve as radiation detectors by producing light when they absorb ionizing radiation.
Some other properties of lanthanum are exploited in a variety of applications. Many lanthanum compounds can be induced to emit electrons when treated with heat in a process called thermionic emission. Lanthanum boride crystals are used as electron emission sources for electron microscopes and ion thrusters used in spacecraft. The thermionic emission of electrons of lanthanum compounds is also exploited in carbon arc lamps, in which lanthanum compounds are sometimes components of one of the electrodes. Lanthanum also binds phosphates in solution. Some water treatment products use this property to remove the free phosphates that feed algae, while the drug lanthanum carbonate uses it to absorb excess phosphate in the blood of patients in end-stage kidney failure. Lanthanum catalysts are widely used in the industrial process of refining petroleum for fuel. Additionally, lanthanum catalysts are being investigated for use in many other processes, including photocatalytic hydrogen production and production of syngas from methane.
Lanthanum is often a component of hydrogen sponge alloys--metals that can absorb and store up to 400 times their own volume of hydrogen gas. These alloys are most commonly used as the negative electrode of nickel-metal hydride (NiMH) batteries. NiMH batteries are rechargeable batteries with high storage capacity and are important for the development of green technologies such as electric vehicles.
Many lanthanum-containing thin film compositions have been investigated in the search for high-k gate dielectrics for use in integrated circuits. Silicon dioxide gate dielectrics have been standard for decades, but the industry has reached the limit of thinness for silicon layers that can serve as an effective gate, and the development of alternate materials could theoretically allow for further miniaturization of microelectronics.
Lanthanum is a light rare earth most commonly obtained by processing the rare earth minerals monazite and bastnasite.
Products
 Lanthanum-rich lanthanide compositions have been used extensively for cracking reactions in FCC catalysts, especially to manufacture low-octane fuel for heavy crude oil. It is utilized in green phosphors based on the phosphate (La0.4Ce0.45Tb0.15)PO4. Lanthanide zirconates and lanthanum strontium manganites are used for their catalytic and conductivity properties and lanthanum stabilized zirconia has useful electrical and mechanical properties. Lanthanum's ability to bind with phosphates in water creates numerous uses in water treatment. It is utilized in laser crystals based on the yttrium-lanthanum-fluoride (YLF) composition. Lanthanum metal is predominantly used in the production of mischmetal and steel additives, but is also
Lanthanum-rich lanthanide compositions have been used extensively for cracking reactions in FCC catalysts, especially to manufacture low-octane fuel for heavy crude oil. It is utilized in green phosphors based on the phosphate (La0.4Ce0.45Tb0.15)PO4. Lanthanide zirconates and lanthanum strontium manganites are used for their catalytic and conductivity properties and lanthanum stabilized zirconia has useful electrical and mechanical properties. Lanthanum's ability to bind with phosphates in water creates numerous uses in water treatment. It is utilized in laser crystals based on the yttrium-lanthanum-fluoride (YLF) composition. Lanthanum metal is predominantly used in the production of mischmetal and steel additives, but is also important in the production of hydrogen storage alloys for nickel-metal hydride (NiMH) batteries. Lanthanum hydride is made inside of pressure chambers and currently holds the new record for high temperature superconductivity at -23 degrees Celsius, which is about 50 degrees more than the previous record. This property allows electrons to flow through the metal hydride with zero resistance.
important in the production of hydrogen storage alloys for nickel-metal hydride (NiMH) batteries. Lanthanum hydride is made inside of pressure chambers and currently holds the new record for high temperature superconductivity at -23 degrees Celsius, which is about 50 degrees more than the previous record. This property allows electrons to flow through the metal hydride with zero resistance.  Lanthanum is available as metal and compound forms with purities ranging from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Lanthanum oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Lanthanum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Lanthanum is available as metal and compound forms with purities ranging from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Lanthanum oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Lanthanum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Lanthanum Properties
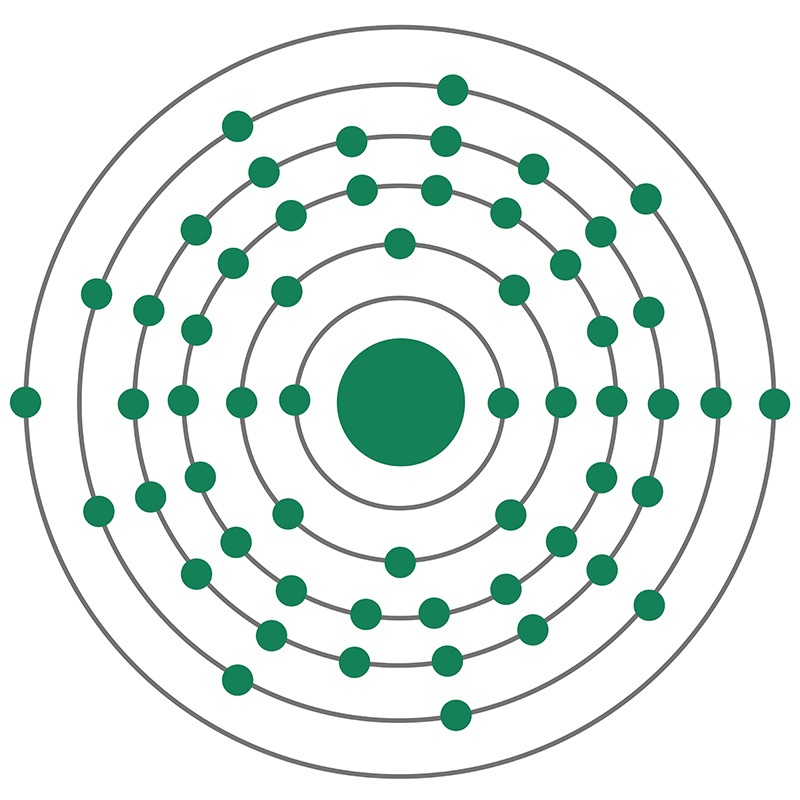
 Lanthanum is a Block F, Group 3, Period 6 element. The number of electrons in each of lanthanum's shells is 2, 8, 18, 18, 9, 2 and its electron configuration is [Xe] 5d1 6s2. The lanthanum atom has a radius of 187.pm and its Van der Waals radius is 240.pm.
Lanthanum is a Block F, Group 3, Period 6 element. The number of electrons in each of lanthanum's shells is 2, 8, 18, 18, 9, 2 and its electron configuration is [Xe] 5d1 6s2. The lanthanum atom has a radius of 187.pm and its Van der Waals radius is 240.pm.  In its elemental form, CAS 7439-91-0, lanthanum has a silvery white appearance. Lanthanum is the first element in the rare earth or lanthanide series. It is the model for all the other trivalent rare earths and it is the second most abundant of the rare earths after cerium. Lanthanum is found in monazite and bastnasite. Lanthanum was first discovered by Carl Mosander in 1839. The name lanthanum originates from the Greek word Lanthaneia which means 'to lie hidden'.
In its elemental form, CAS 7439-91-0, lanthanum has a silvery white appearance. Lanthanum is the first element in the rare earth or lanthanide series. It is the model for all the other trivalent rare earths and it is the second most abundant of the rare earths after cerium. Lanthanum is found in monazite and bastnasite. Lanthanum was first discovered by Carl Mosander in 1839. The name lanthanum originates from the Greek word Lanthaneia which means 'to lie hidden'.
Health, Safety & Transportation Information for Lanthanum
Lanthanum is somewhat toxic. Safety data for Lanthanum and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Lanthanum
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H260 |
| Hazard Codes | N/A |
| Risk Codes | N/A |
| Safety Precautions | N/A |
| RTECS Number | N/A |
| Transport Information | UN 3208 4.3/PG 1 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Lanthanum Isotopes
Naturally occurring lanthanum (La) has one stable isotope: 139La.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 117La | 116.95007(43)# | 23.5(26) ms | ß+ to 117Ba; p to 116Cs | (3/2+,3/2-) | N/A | 927.83 | - |
| 118La | 117.94673(32)# | 200# ms | ß+ to 118Ba | N/A | N/A | 945.23 | - |
| 119La | 118.94099(43)# | 1# s | ß+ to 119Ba | 11/2-# | N/A | 953.31 | - |
| 120La | 119.93807(54)# | 2.8(2) s | ß+ to 120Ba; ß+ + p to 119Cs | N/A | N/A | 970.7 | - |
| 121La | 120.93301(54)# | 5.3(2) s | ß+ to 121Ba; ß+ + p to 120Cs | 11/2-# | N/A | 978.78 | - |
| 122La | 121.93071(32)# | 8.6(5) s | ß+ to 122Ba; ß+ + p to 121Cs | N/A | N/A | 986.86 | - |
| 123La | 122.92624(21)# | 17(3) s | ß+ to 123Ba | 11/2-# | N/A | 1004.25 | - |
| 124La | 123.92457(6) | 29.21(17) s | ß+ to 124Ba | (7-,8-) | N/A | 1012.33 | - |
| 125La | 124.920816(28) | 64.8(12) s | ß+ to 125Ba | (11/2-) | N/A | 1020.41 | - |
| 126La | 125.91951(10) | 54(2) s | ß+ to 126Ba | (5)(+#) | N/A | 1037.81 | - |
| 127La | 126.916375(28) | 5.1(1) min | ß+ to 127Ba | (11/2-) | N/A | 1045.89 | - |
| 128La | 127.91559(6) | 5.18(14) min | ß+ to 128Ba | (5+) | N/A | 1053.96 | - |
| 129La | 128.912693(22) | 11.6(2) min | ß+ to 129Ba | 3/2+ | N/A | 1062.04 | - |
| 130La | 129.912369(28) | 8.7(1) min | ß+ to 130Ba | 3(+) | N/A | 1070.12 | - |
| 131La | 130.91007(3) | 59(2) min | ß+ to 131Ba | 3/2+ | N/A | 1078.2 | - |
| 132La | 131.91010(4) | 4.8(2) h | EC to 132Ba | 2- | N/A | 1086.28 | - |
| 133La | 132.90822(3) | 3.912(8) h | EC to 133Ba | 5/2+ | N/A | 1103.67 | - |
| 134La | 133.908514(21) | 6.45(16) min | EC to 134Ba | 1+ | N/A | 1111.75 | - |
| 135La | 134.906977(11) | 19.5(2) h | EC to 135Ba | 5/2+ | N/A | 1119.83 | - |
| 136La | 135.90764(6) | 9.87(3) min | EC to 136Ba | 1+ | N/A | 1127.91 | - |
| 137La | 136.906494(14) | 6(2)E+4 y | EC to 137Ba | 7/2+ | 2.7 | 1135.99 | - |
| 138La | 137.907112(4) | 1.02(1)E+11 y | ß+ to 138Ba; ß- to 138Ce | 5+ | 3.7139 | 1144.07 | 0.09 |
| 139La | 138.9063533(26) | STABLE | - | 7/2+ | 2.7832 | 1152.15 | 99.91 |
| 140La | 139.9094776(26) | 1.6781(3) d | ß- to 140Ce | 3- | N/A | 1160.22 | - |
| 141La | 140.910962(5) | 3.92(3) h | ß- to 141Ce | (7/2+) | N/A | 1158.99 | - |
| 142La | 141.914079(6) | 91.1(5) min | ß- to 142Ce | 2- | N/A | 1167.07 | - |
| 143La | 142.916063(17) | 14.2(1) min | ß- to 143Ce | (7/2)+ | N/A | 1175.14 | - |
| 144La | 143.91960(5) | 40.8(4) s | ß- to 144Ce | (3-) | N/A | 1183.22 | - |
| 145La | 144.92165(10) | 24.8(20) s | ß- to 145Ce; ß- + n to 144Ce | (5/2+) | N/A | 1181.99 | - |
| 146La | 145.92579(8) | 6.27(10) s | ß- to 146Ce; ß- + n to 146Ce | 2- | N/A | 1190.06 | - |
| 147La | 146.92824(5) | 4.015(8) s | ß- to 147Ce; ß- + n to 146Ce | (5/2+) | N/A | 1198.14 | - |
| 148La | 147.93223(6) | 1.26(8) s | ß- to 148Ce; ß- + n to 147Ce | (2-) | N/A | 1196.91 | - |
| 149La | 148.93473(34)# | 1.05(3) s | ß- to 149Ce; ß- + n to 148Ce | 5/2+# | N/A | 1204.98 | - |
| 150La | 149.93877(43)# | 510(30) ms | ß- to 150Ce; ß- + n to 149Ce | (3+) | N/A | 1213.06 | - |
| 151La | 150.94172(43)# | 300# ms [>300 ns] | ß- to 151Ce | 5/2+# | N/A | 1211.82 | - |
| 152La | 151.94625(43)# | 200# ms [>300 ns] | ß- to 152Ce | N/A | N/A | 1219.9 | - |
| 153La | 152.94962(64)# | 150# ms [>300 ns] | ß- to 153Ce | 5/2+# | N/A | 1227.98 | - |
| 154La | 153.95450(64)# | 100# ms | ß- to 154Ce | N/A | N/A | 1226.74 | - |
| 155La | 154.95835(86)# | 60# ms | ß- to 155Ce | 5/2+# | N/A | 1234.82 | - |