About Hafnium
In 1869, Dmitri Mendeleev’s periodic table predicted the existence of an element with 72 protons that would be chemically similar to zirconium . Though Henry Moseley and Niels Bohr’s subsequent theoretical models of the elements supported this prediction, it was not until 1923 that Georg von Hevesy and Dirk Coster provided the first empirical evidence of the elusive element 72, the penultimate element with stable isotopes to be discovered (followed by rhenium two years later). While working at the Bohr Institute of Theoretical Physics in Copenhagen, the two chemists identified the new element via x-ray spectroscopy analysis of a zirconium ore and named it "hafnium" after Hafnia, the Latin name for Copenhagen.
True to Mendeleev’s theory, Hafnium is extremely similar to zirconium; with nearly identical atomic radii, the two always occur together in nature in a continuous solid-solution and are two of the most difficult elements to separate. Primarily zirconium-based minerals typically contain 1-3% hafnium, the most common (and the subject of Coster and von Hevesy’s experiment) being zircon (zirconium silicate) with up to 4% hafnium by content. Other such zirconium-rich minerals include eudialyte, thortveitite, cyrtolite, armstrongite, alvite, and hafnon; the two elements can also be found in minerals such as the titanium ores ilmenite and rutile. Hafnium metal is primarily obtained as a byproduct of producing high-purity nuclear grade zirconium metal. The metals are typically chemically separated via a liquid-liquid extraction process that utilizing the slightly different solubilities of the two metals’ salts.
In its elemental form, hafnium is a ductile gray metal with a brilliantly lustrous silver sheen. Exposure to air causes the metal to form an impenetrable oxide film on its surface which lends the metal an extremely high resistance to corrosion and attack by most acids and alkalis. Because Hafnium’s melting point is high among its fellow transition metals, it is occasionally classified as a refractory metal. Like many other metals, hafnium as a fine powder is pyrophoric, meaning that it can ignite in air; for this reason, it is considered a hazardous material despite being non-toxic to humans. Thought hafnium shares many chemical and physical properties with zirconium, the two metals differ significantly in their densities (hafnium being roughly twice as dense) and their nuclear properties. Hafnium is an excellent neutron absorber with a high thermal neutron cross section, about 600 times that of zirconium, and one of its primary commercial uses is in control rods of nuclear reactors. It has also been used to enhance radiotherapy in the treatment of cancer.
Compound and alloy forms of hafnium are notable for their refractory properties. The melting points of several hafnium compounds are unparalleled within their respective groups: hafnium nitride’s (3310 °C) is the highest of any nitride, hafnium carbide’s (3890 °C) is the highest of any known binary compound, and tantalum hafnium carbide’s (4215 °C) is the single highest of any known compound. Thus, forms of hafnium are frequently employed in high-temperature environments as components of furnace linings, ceramics, rocket thrusters and jet engines for the aerospace industry, nozzle tips for plasma arc cutting, and wear resistant coatings. High performance superalloys typically contain hafnium in combination with metals like titanium, tungsten, and niobium; the metal improves creep ductility, strengthens grain boundaries, and increases corrosion resistance. Other applications for hafnium include serving as an oxygen and nitrogen “getter” in vacuum tubes and incandescent lighting, in geological dating (as isotopes), and in organic catalysis. Additionally, compounds like hafnium oxide and hafnium silicate have shown great promise as high-k dielectric materials that increase efficiency of semiconductor devices such as integrated circuits and transistors in the field of advanced microelectronics.
Products
There are relatively few technical uses for hafnium and, due to its ability as a nuclear "getter" or absorber of neutrons, much of the hafnium that is produced is used in control rods for nuclear reactors. Hafnium is also used in iron, titanium, niobium, tantalum, and other alloys. Hafnium is replacing polysilicon as the principle gate or electrode material in metal-oxide semiconductor field effect transistors (MOSFETs), which are the basis for all modern semiconductors. As semiconductors get smaller, the limiting factor in further size reduction has been the ability of the silicon dioxide gate to perform below 10 angstroms where leakage occurs.  Recent research has been devoted to the development of high-k materials which can function as a di-electric barrier or gate with lower leakage. Using hafnium based alloys as this di-electric gate has allowed for the development of MOSFET gates smaller than 10 angstroms. This allows for further size reduction, reduced switching power requirements and improved performance.
Recent research has been devoted to the development of high-k materials which can function as a di-electric barrier or gate with lower leakage. Using hafnium based alloys as this di-electric gate has allowed for the development of MOSFET gates smaller than 10 angstroms. This allows for further size reduction, reduced switching power requirements and improved performance.  Hafnium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms of hafnium include pellets, rod, wire and granules for evaporation source material purposes. Hafnium nanomaterials provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Hafnium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Hafnium is also available in soluble forms including chloride, nitrate and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
Hafnium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms of hafnium include pellets, rod, wire and granules for evaporation source material purposes. Hafnium nanomaterials provide ultra-high surface area which nanotechnology research and recent experiments demonstrate function to create new and unique properties and benefits. Hafnium oxide is available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Hafnium is also available in soluble forms including chloride, nitrate and acetate. These compounds can be manufactured as solutions at specified stoichiometries.
Hafnium Properties
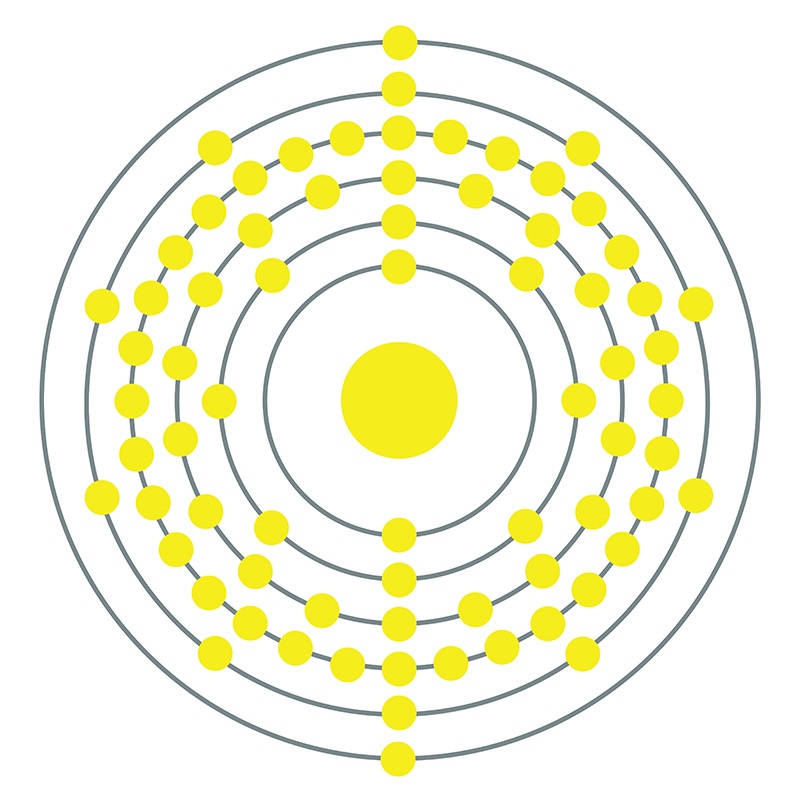
 Hafnium is a Block D, Group 4, Period 6 element. The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electronic configuration is [Xe] 4f14 5d2 6s2.
Hafnium is a Block D, Group 4, Period 6 element. The number of electrons in each of Hafnium's shells is 2, 8, 18, 32, 10, 2 and its electronic configuration is [Xe] 4f14 5d2 6s2. 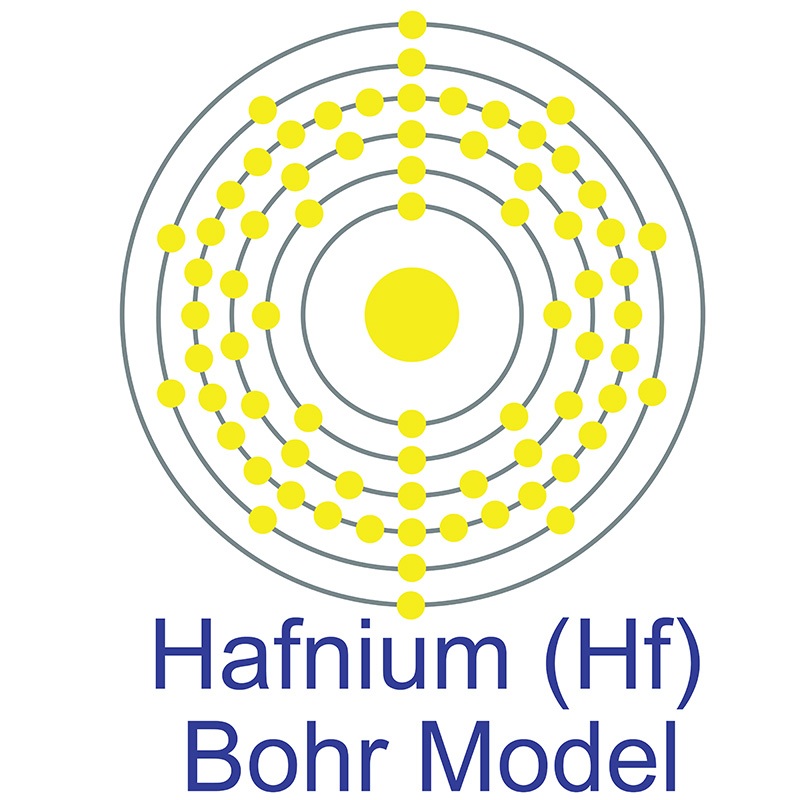 In its elemental form, CAS 7440-58-6, hafnium has a steel gray appearance. Hafnium does not exist as a free element in nature. It is found in zirconium compounds such as zircon (ZrSiO4). Hafnium was first predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy.
In its elemental form, CAS 7440-58-6, hafnium has a steel gray appearance. Hafnium does not exist as a free element in nature. It is found in zirconium compounds such as zircon (ZrSiO4). Hafnium was first predicted by Dmitri Mendeleev in 1869 but it was not until 1922 that it was first isolated Dirk Coster and George de Hevesy.
Health, Safety & Transportation Information for Hafnium
Hafnium is not toxic; however, safety data for hafnium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Magnesium.
| Safety Data | |
|---|---|
| Material Safety Data Sheet | MSDS |
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | N/A |
| RTECS Number | MG4600000 |
| Transport Information | N/A |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Hafnium Isotopes
Naturally occurring hafnium (Hf) has five stable isotopes: 176Hf, 177Hf, 178Hf, 179Hf, and 180Hf.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 153Hf | 152.97069(54)# | 400# ms [>200 ns] | Unknown | 1/2+# | N/A | 1183.2 | - |
| 154Hf | 153.96486(54)# | 2(1) s | ß+ to 154Lu; a to 150Yb | 0+ | N/A | 1200.6 | - |
| 155Hf | 154.96339(43)# | 890(120) ms | ß+ to 155Lu; a to 151Yb | 7/2-# | N/A | 1208.68 | - |
| 156Hf | 155.95936(22) | 23(1) ms | a to 152Yb; ß+ to 156Lu | 0+ | N/A | 1226.07 | - |
| 157Hf | 156.95840(21)# | 115(1) ms | a to 153Yb; ß+ to 157Lu | 7/2- | N/A | 1234.15 | - |
| 158Hf | 157.954799(19) | 2.84(7) s | ß+ to 158Lu; a to 154Yb | 0+ | N/A | 1242.23 | - |
| 159Hf | 158.953995(18) | 5.20(10) s | ß+ to 159Lu; a to 155Yb | 7/2-# | N/A | 1250.31 | - |
| 160Hf | 159.950684(12) | 13.6(2) s | ß+ to 160Lu; a to 156Yb | 0+ | N/A | 1258.39 | - |
| 161Hf | 160.950275(24) | 18.2(5) s | ß+ to 161Lu; a to 157Yb | 3/2-# | N/A | 1266.46 | - |
| 162Hf | 161.94721(1) | 39.4(9) s | ß+ to 162Lu; a to 158Yb | 0+ | N/A | 1283.86 | - |
| 163Hf | 162.94709(3) | 40.0(6) s | ß+ to 163Lu; a to 159Yb | 3/2-# | N/A | 1291.94 | - |
| 164Hf | 163.944367(22) | 111(8) s | ß+ to 164Lu | 0+ | N/A | 1300.02 | - |
| 165Hf | 164.94457(3) | 76(4) s | ß+ to 165Lu | (5/2-) | N/A | 1308.1 | - |
| 166Hf | 165.94218(3) | 6.77(30) min | ß+ to 166Lu | 0+ | N/A | 1316.17 | - |
| 167Hf | 166.94260(3) | 2.05(5) min | ß+ to 167Lu | (5/2)- | N/A | 1324.25 | - |
| 168Hf | 167.94057(3) | 25.95(20) min | ß+ to 168Lu | 0+ | N/A | 1332.33 | - |
| 169Hf | 168.94126(3) | 3.24(4) min | ß+ to 169Lu | (5/2)- | N/A | 1340.41 | - |
| 170Hf | 169.93961(3) | 16.01(13) h | EC to 170Lu | 0+ | N/A | 1357.81 | - |
| 171Hf | 170.94049(3) | 12.1(4) h | ß+ to 170Lu | 7/2(+) | N/A | 1356.57 | - |
| 172Hf | 171.939448(26) | 1.87(3) y | EC to 172Lu | 0+ | N/A | 1373.96 | - |
| 173Hf | 172.94051(3) | 23.6(1) h | EC to 173Lu | 1/2- | N/A | 1372.73 | - |
| 174Hf | 173.940046(3) | 2.0(4)E+15 y | a to 170Yb | 0+ | N/A | 1380.8 | 0.16 |
| 175Hf | 174.941509(3) | 70(2) d | EC to 175Lu | 5/2- | 0.54 | 1388.88 | - |
| 176Hf | 175.9414086(24) | Observationally Stable | - | 0+ | N/A | 1396.96 | 5.26 |
| 177Hf | 176.9432207(23) | Observationally Stable | - | 7/2- | 0.7936 | 1405.04 | 18.6 |
| 178Hf | 177.9436988(23) | Observationally Stable | - | 0+ | N/A | 1413.12 | 27.28 |
| 179Hf | 178.9458161(23) | Observationally Stable | - | 9/2+ | -0.6409 | 1421.2 | 13.62 |
| 180Hf | 179.9465500(23) | Observationally Stable | - | 0+ | N/A | 1429.28 | 35.08 |
| 181Hf | 180.9491012(23) | 42.39(6) d | ß- to 181Ta | 1/2- | N/A | 1437.36 | - |
| 182Hf | 181.950554(7) | 8.90(9)E+6 y | ß- to 182Ta | 0+ | N/A | 1436.12 | - |
| 183Hf | 182.95353(3) | 1.067(17) h | ß- to 183Ta | (3/2-) | N/A | 1444.2 | - |
| 184Hf | 183.95545(4) | 4.12(5) h | ß- to 184Ta | 0+ | N/A | 1452.28 | - |
| 185Hf | 184.95882(21)# | 3.5(6) min | ß- to 185Ta | 3/2-# | N/A | 1460.35 | - |
| 186Hf | 185.96089(32)# | 2.6(12) min | ß- to 186Ta | 0+ | N/A | 1459.12 | - |
| 187Hf | 186.96459(43)# | 30# s [>300 ns] | Unknown | N/A | N/A | 1467.19 | - |
| 188Hf | 187.96685(54)# | 20# s [>300 ns] | Unknown | 0+ | N/A | 1475.27 | - |