About Germanium
When Mendeleev published his first Periodic Table of the Elements in 1869, he included several as-yet undiscovered elements. He gave each a temporary name and predicted its properties based on the surrounding elements. One of these was named ekasilicon, and Mendeleev almost perfectly predicted the properties of the actual element, which was discovered by Clemens Winkler in 1886 and named after his native Germany: thus, germanium.
Germanium is a semiconducting metalloid with properties similar to silicon, and its use in electronic devices actually pre-dated the use of the more famous element. The first transistors were produced from germanium in Bell labs in 1947. Ultimately, the technologies available for use with silicon and the abundance of silicon led to many more semiconductor applications using silicon, including standard computer chips, and for many years germanium played a limited role in semiconductor devices. However, today new technologies are again making germanium a key material for electronics applications. Germanium is preferable to silicon in some types of photovoltaic cells used to harvest solar energy, and is used as a key substrate material in production of high-brightness LEDs for flashlights, automobile tail lights, cameras, traffic signals, and display screens. As an LED component, germanium is sometimes preferable to the alternative, gallium arsenide, as it breaks less frequently and poses fewer disposal issues. Additionally, microchip designs using germanium-on-insulator or silicon-germanium technology are seeing increased use.
Another key use of germanium comes from the favorable optical properties of it and some of its compounds. Germanium oxide has a high index of refraction and low optical dispersion, making it appropriate for use in wide-angle lenses and some microscopes. Germanium oxide also imparts some of these properties when used as a dopant in silica glass, and is used as such in the core of optical fibers. An additional useful optical property is that germanium glass is transparent to infrared radiation. It is therefore used in thermal imaging cameras, night vision systems, and sensitive infrared detectors. Another use of germanium in optical systems is in the material germanium-antimony-tellurium, or GeSbTe, a phase change material used in rewritable optical disks (CD-RW, DVD-RW) and other phase change memory devices.
In addition to its electronic and optical applications, germanium also finds uses in a few other key areas. Germanium oxide is used as a catalyst in the making of many plastics. When germanium is added in small amounts to sterling silver, it reduces firescale and tarnish, and makes the final metal harder. Contrary to past beliefs, germanium has not been shown to have any medical function and is considered potentially hazardous if consumed; nonetheless some nutritional supplements contain the element.
Though germanium is not particularly rare, it is not contained in any mineral in large enough percentages to be worth mining for germanium specifically. Instead, germanium is derived from concentrates produced as byproducts of mining for other metals, particularly zinc, and is additionally recovered from the fly ash of some coal power plants.
Germanium is a very important semiconductor and is also finding many other applications including use as an alloying agent, as a phosphor in  fluorescent lamps, and as a catalyst. Germanium and germanium oxide are transparent to the infrared and are used in infrared spectroscopes
fluorescent lamps, and as a catalyst. Germanium and germanium oxide are transparent to the infrared and are used in infrared spectroscopes  and other optical equipment, including extremely sensitive infrared detectors. The high refractive index and dispersion properties of its oxides have made germanium useful as a component of wide-angle camera lenses and microscope objectives. Elemental or metallic forms of Germanium include pellets, rod, wire and granules for evaporation source material purposes. Germanium oxide is available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Germanium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
and other optical equipment, including extremely sensitive infrared detectors. The high refractive index and dispersion properties of its oxides have made germanium useful as a component of wide-angle camera lenses and microscope objectives. Elemental or metallic forms of Germanium include pellets, rod, wire and granules for evaporation source material purposes. Germanium oxide is available in forms including powders and dense pellets for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Fluorides are another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Germanium is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Germanium Properties
 Germanium is a Block P, Group 14, Period 4 element. The number of electrons in each of germanium's shells is 2, 8, 18, 4 and its electron configuration is [Ar] 3d10 4s2 4p2.
Germanium is a Block P, Group 14, Period 4 element. The number of electrons in each of germanium's shells is 2, 8, 18, 4 and its electron configuration is [Ar] 3d10 4s2 4p2. 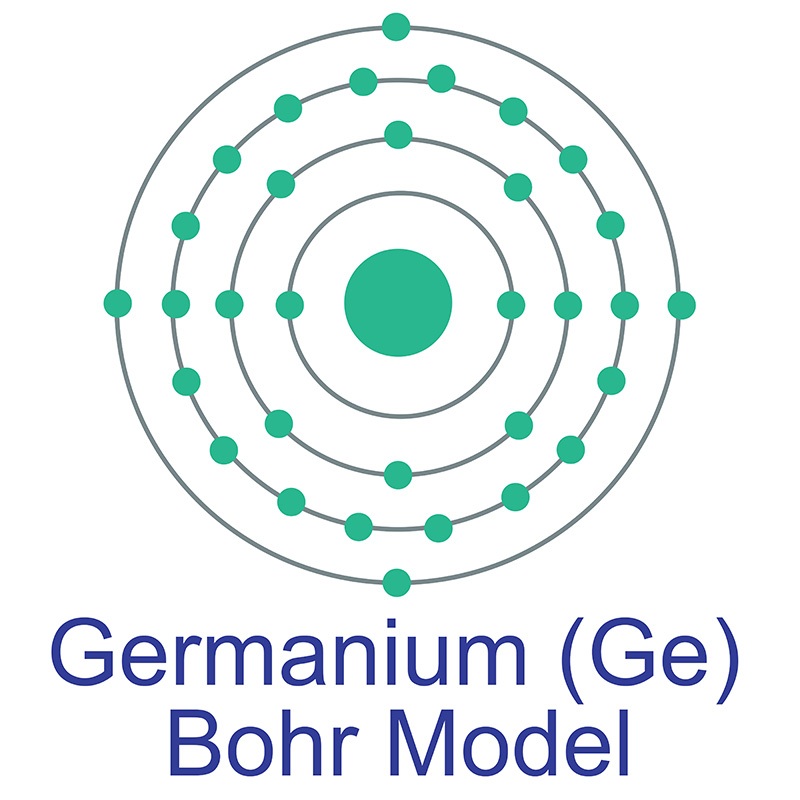 The germanium atom has a radius of 122.5.pm and its Van der Waals radius is 211.pm. In its elemental form, CAS 7440-56-4, germanium is a brittle grayish white semi-metallic element. Germanium is too reactive to be found naturally on Earth in its native state. It is commercially obtained from zinc
The germanium atom has a radius of 122.5.pm and its Van der Waals radius is 211.pm. In its elemental form, CAS 7440-56-4, germanium is a brittle grayish white semi-metallic element. Germanium is too reactive to be found naturally on Earth in its native state. It is commercially obtained from zinc  ores and certain coals. It is also found in argyrodite and germanite. Germanium was first discovered by Clemens Winkler in 1886. The name Germanium originates from the Latin word "Germania" meaning "Germany".
ores and certain coals. It is also found in argyrodite and germanite. Germanium was first discovered by Clemens Winkler in 1886. The name Germanium originates from the Latin word "Germania" meaning "Germany".
Health, Safety & Transportation Information for Germanium
Germanium is not toxic in its elemental form; however, safety data for Germanium metal, nanoparticles and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific Germanium material or compound referenced in the Products tab.
| Safety Data | |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Hazard Codes | Xi |
| Risk Codes | 36/37/38 |
| Safety Precautions | 26-36/39 |
| RTECS Number | LY5200000 |
| Transport Information | N/A |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Germanium Isotopes
Germanium (Ge) has five naturally occurring isotopes, 70Ge, 72Ge, 73Ge, 74Ge, and 76Ge.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 58Ge | 57.99101(34)# | Unknown | 2p to 56Zn | 0+ | N/A | 441.04 | - |
| 59Ge | 58.98175(30)# | Unknown | 2p to 57Zn | 7/2-# | N/A | 458.44 | - |
| 60Ge | 59.97019(25)# | 30# ms | ß+ to 60Ga; 2p to 58Zn | 0+ | N/A | 476.77 | - |
| 61Ge | 60.96379(32)# | 39(12) ms | ß+ + p to 60Zn; ß+ to 61Ga | (3/2-)# | N/A | 491.37 | - |
| 62Ge | 61.95465(15)# | 129(35) ms | ß+ to 62Ga | 0+ | N/A | 507.83 | - |
| 63Ge | 62.94964(21)# | 142(8) ms | ß+ to 63Ga | (3/2-)# | N/A | 520.57 | - |
| 64Ge | 63.94165(3) | 63.7(25) s | EC to 64Ga | 0+ | N/A | 536.1 | - |
| 65Ge | 64.93944(11) | 30.9(5) s | EC to 65Ga | (3/2)- | N/A | 546.04 | - |
| 66Ge | 65.93384(3) | 2.26(5) h | EC to 66Ga | 0+ | N/A | 559.71 | - |
| 67Ge | 66.932734(5) | 18.9(3) min | EC to 67Ga | 1/2- | N/A | 568.72 | - |
| 68Ge | 67.928094(7) | 270.95(16) d | EC to 68Ga | 0+ | N/A | 580.52 | - |
| 69Ge | 68.9279645(14) | 39.05(10) h | EC to 69Ga | 5/2- | 0.735 | 589.53 | - |
| 70Ge | 69.9242474(11) | STABLE | - | 0+ | N/A | 600.41 | 20.84 |
| 71Ge | 70.9249510(11) | 11.43(3) d | EC to 71Ga | 1/2- | 0.547 | 608.49 | - |
| 72Ge | 71.9220758(18) | STABLE | - | 0+ | N/A | 618.43 | 27.54 |
| 73Ge | 72.9234589(18) | STABLE | - | 9/2+ | -0.8794669 | 625.58 | 7.73 |
| 74Ge | 73.9211778(18) | STABLE | - | 0+ | N/A | 635.52 | 36.28 |
| 75Ge | 74.9228589(18) | 82.78(4) min | ß- to 75As | 1/2- | 0.51 | 642.66 | - |
| 76Ge | 75.9214026(18) | 1.78(8)E+21 y | 2ß- to 76Se | 0+ | N/A | 651.67 | 7.61 |
| 77Ge | 76.9235486(18) | 11.30(1) h | ß- to 77As | 7/2+ | N/A | 657.89 | - |
| 78Ge | 77.922853(4) | 88(1) min | ß- to 78As | 0+ | N/A | 666.9 | - |
| 79Ge | 78.9254(1) | 18.98(3) s | ß- to 79As | (1/2)- | N/A | 672.18 | - |
| 80Ge | 79.92537(3) | 29.5(4) s | ß- to 80As | 0+ | N/A | 680.26 | - |
| 81Ge | 80.92882(13) | 7.6(6) s | ß- to 81As | 9/2+# | N/A | 685.55 | - |
| 82Ge | 81.92955(26) | 4.55(5) s | ß- to 82As | 0+ | N/A | 692.69 | - |
| 83Ge | 82.93462(21)# | 1.85(6) s | ß- to 83As | (5/2+)# | N/A | 696.11 | - |
| 84Ge | 83.93747(32)# | 0.947(11) s | ß- to 84As; ß- + n to 83As | 0+ | N/A | 701.4 | - |
| 85Ge | 84.94303(43)# | 535(47) ms | ß- to 85As; ß- + n to 84As | 5/2+# | N/A | 703.89 | - |
| 86Ge | 85.94649(54)# | >150 ns | ß- + n to 85As; ß- to 86As | 0+ | N/A | 709.17 | - |
| 87Ge | 86.95251(54)# | 0.14# s | Unknown | 5/2+# | N/A | 711.66 | - |
| 88Ge | 87.95691(75)# | >=300 ns | Unknown | 0+ | N/A | 716.01 | - |
| 89Ge | 88.96383(97)# | >150 ns | Unknown | 3/2+# | N/A | 717.57 | - |