About Tellurium
Tellurium was discovered in Franz-Joseph Müller von Reichenstein in 1782 when he isolated it as a trace component from a sample of gold ore. He initially believed his find to be antimony, but eventually realized its properties did not match any known element and reported his findings to other chemists. In 1798, Martin Heinrich Klaproth completed further tests to confirm that a new element had in fact been discovered, and named it “tellurium”, meaning “earth.”
Tellurium is a brittle, silver-white, semiconducting metalloid that also exhibits mild photoconductivity. One of the largest uses for tellurium are in semiconducting compound materials that exploit its unique electrical properties. Cadmium telluride is particularly well known as the basis for a type of thin film solar cell that can be manufactured with relatively little impact on the environment and used in some installations at a comparable cost to traditional silicon cells. Cadmium zinc telluride is likewise used in solar cells, as well as in radiation detectors, terahertz wave generation and detection devices, electro-optic modulators, solid-state x-ray detectors, and photoreactive gratings. When tellurium is added as a dopant to zinc selenide, the resultant material is a scintillator material used in x-ray and gamma ray detectors. Bismuth telluride and lead telluride are both thermoelectric materials used in thermoelectric refrigeration and in portable thermal generators. Additionally, tellurium’s photoconductivity once lent it to use along with the similar element selenium in photocopiers, however modern photocopiers use organic photoconductors in place of these elements.
Tellurium also plays a significant role in technology in the form of chalcogenide glasses. These glasses, made using sulfur, selenium, or tellurium compounds, exhibit high refractive indices and non-linear optical effects, and are frequently used in optical fibers for telecommunications, lasers, photonic integrated circuits, and other optical applications. Additionally, some chalcogenide glasses including GeSbTe and AgInSbTe undergo predictable changes in crystal structure driven by thermal energy, and this property is exploited in rewritable optical disks and phase-change computer memory.
Another major use of tellurium is as an additive to metal alloys. It is used primarily to improve the machinability of steel or copper, and to improve strength and durability of lead. It is also found in some forms of cast iron. Additionally, telluride compounds may be used as pigments to color ceramics, as components of blasting caps, or as catalysts for some industrial chemical processes.
Tellurium is a relatively rare element, and is produced commercially mostly from byproducts of electrolytic copper refining. Tellurium is also occasionally recovered from old devices which contained it, most often outdated photocopiers.
Products
Tellurium is a p-type semiconductor, and shows greater conductivity in certain directions, depending on alignment of the atoms. Many tellurium compounds exhibit photoconductivity--their conductivity increases slightly with exposure to light--which makes many tellurides candidates for solar energy applications. Tellurium improves the machinability of copper and stainless steel, and its addition to lead improves its strength and hardness.  Tellurium is used as a basic ingredient in blasting caps, and is added to cast iron for chill control. Tellurium is used in ceramics. Bismuth telluride has been used in thermoelectric devices.
Tellurium is used as a basic ingredient in blasting caps, and is added to cast iron for chill control. Tellurium is used in ceramics. Bismuth telluride has been used in thermoelectric devices.  Tellurium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Tellurium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Tellurium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tellurium is also available in soluble forms including chlorides and nitrates. These compounds can be manufactured as solutions at specified stoichiometries.
Tellurium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity). Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Tellurium oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Tellurium fluoride is another insoluble form for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Tellurium is also available in soluble forms including chlorides and nitrates. These compounds can be manufactured as solutions at specified stoichiometries.
Tellurium Properties
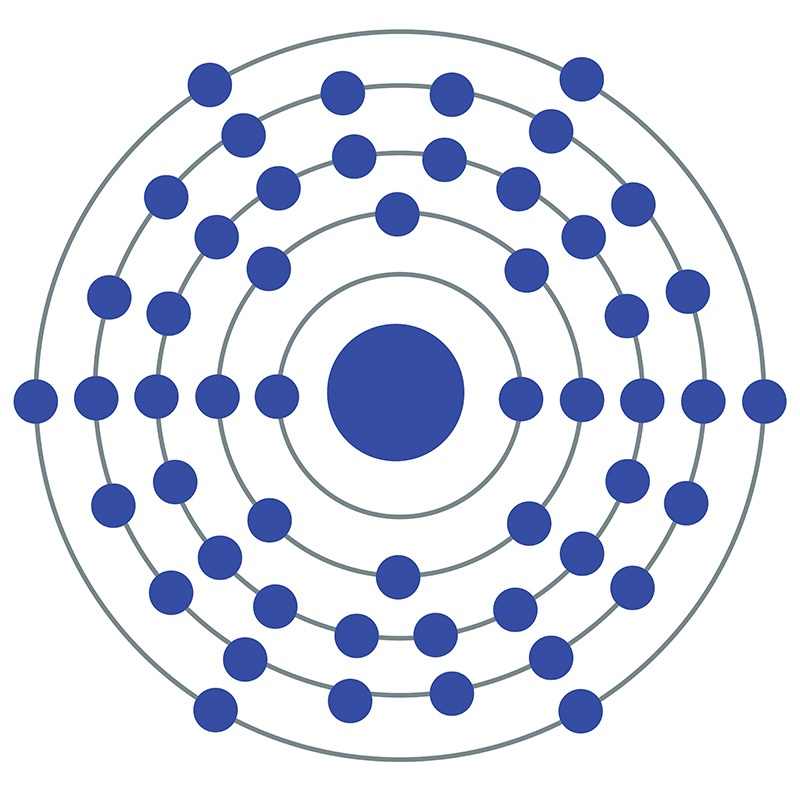
 Tellurium is a Block P, Group 16, Period 5 element. The number of electrons in each of Tellurium's shells is 2, 8, 18, 18, 6 and its electron configuration is
Tellurium is a Block P, Group 16, Period 5 element. The number of electrons in each of Tellurium's shells is 2, 8, 18, 18, 6 and its electron configuration is  [Kr] 4d10 5s2 5p4. In its elemental form tellurium's CAS number is 13494-80-9. The tellurium atom has a radius of 140.pm and its Van der Waals radius is 206.pm. Tellurium is most commonly sourced from the anode sludges produced as a byproduct of copper refining.
[Kr] 4d10 5s2 5p4. In its elemental form tellurium's CAS number is 13494-80-9. The tellurium atom has a radius of 140.pm and its Van der Waals radius is 206.pm. Tellurium is most commonly sourced from the anode sludges produced as a byproduct of copper refining.  Tellurium was first discovered by Franz Muller von Reichenstein in 1782. The name Tellurium originates from the Greek word 'Tellus' meaning Earth.
Tellurium was first discovered by Franz Muller von Reichenstein in 1782. The name Tellurium originates from the Greek word 'Tellus' meaning Earth.
Health, Safety & Transportation Information for Tellurium
Safety data for Tellurium and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental Tellurium.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Hazard Codes | T |
| Risk Codes | 25 |
| Safety Precautions | 45 |
| RTECS Number | WY2625000 |
| Transport Information | UN 3288 6.1/PG 3 |
| WGK Germany | 3 |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Tellurium Isotopes
Tellurium has six stable isotopes: 120Te, 122Te, 123Te, 124Te, 125Te and 126Te.
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 105Te | 104.94364(54)# | 1# µs | Unknown | 5/2+# | N/A | 845.82 | - |
| 106Te | 105.93750(14) | 70(20) µs [70(+20-10) µs] | a to 102Sn | 0+ | N/A | 863.21 | - |
| 107Te | 106.93501(32)# | 3.1(1) ms | a to 103Sn; ß+ to 107Sb | 5/2+# | N/A | 871.29 | - |
| 108Te | 107.92944(11) | 2.1(1) s | ß+ to 108Sb; a to 104Sn; ß+ + p to 107Sb; ß+ + a to 104Ln | 0+ | N/A | 888.68 | - |
| 109Te | 108.92742(7) | 4.6(3) s | ß+ to 109Sb; ß+ + p to 108Sb; a to 105Sn ; ß+ + a to 105Ln | (5/2+) | N/A | 896.76 | - |
| 110Te | 109.92241(6) | 18.6(8) s | ß+ to 110Sb; ß+ + p to 110Sb | 0+ | N/A | 904.84 | - |
| 111Te | 110.92111(8) | 19.3(4) s | ß+ to 111Sb | (5/2)+# | N/A | 912.92 | - |
| 112Te | 111.91701(18) | 2.0(2) min | ß+ to 112Sb | 0+ | N/A | 930.31 | - |
| 113Te | 112.91589(3) | 1.7(2) min | ß+ to 113Sb | (7/2+) | N/A | 938.39 | - |
| 114Te | 113.91209(3) | 15.2(7) min | ß+ to 114Sb | 0+ | N/A | 946.47 | - |
| 115Te | 114.91190(3) | 5.8(2) min | ß+ to 115Sb | 7/2+ | N/A | 954.55 | - |
| 116Te | 115.90846(3) | 2.49(4) h | EC to 116Sb | 0+ | N/A | 971.95 | - |
| 117Te | 116.908645(14) | 62(2) min | EC to 117Sb | 1/2+ | N/A | 980.02 | - |
| 118Te | 117.905828(16) | 6.00(2) d | EC to 118Sb | 0+ | N/A | 988.1 | - |
| 119Te | 118.906404(9) | 16.05(5) h | EC to 119Sb | 1/2+ | 0.25 | 996.18 | - |
| 120Te | 119.90402(1) | Observationally Stable | - | 0+ | N/A | 1004.26 | 0.09 |
| 121Te | 120.904936(28) | 19.16(5) d | EC to 121Sb | 1/2+ | N/A | 1012.34 | - |
| 122Te | 121.9030439(16) | STABLE | - | 0+ | N/A | 1020.42 | 2.55 |
| 123Te | 122.9042700(16) | >600E+12 y | - | 1/2+ | -0.73679 | 1028.5 | 0.89 |
| 124Te | 123.9028179(16) | STABLE | - | 0+ | N/A | 1036.58 | 4.74 |
| 125Te | 124.9044307(16) | Observationally Stable | - | 1/2+ | -0.88828 | 1044.65 | 7.07 |
| 126Te | 125.9033117(16) | STABLE | - | 0+ | N/A | 1052.73 | 18.84 |
| 127Te | 126.9052263(16) | 9.35(7) h | ß- to 127I | 3/2+ | 0.64 | 1060.81 | - |
| 128Te | 127.9044631(19) | 2.2(3)E+24 y | 2ß- to 128Xe | 0+ | N/A | 1068.89 | 31.74 |
| 129Te | 128.9065982(19) | 69.6(3) min | ß- to 129I | 3/2+ | 0.7 | 1076.97 | - |
| 130Te | 129.9062244(21) | 790(100)E+18 y | 2ß- to 130Xe | 0+ | N/A | 1085.05 | - |
| 131Te | 130.9085239(21) | 25.0(1) min | ß- to 131I | 3/2+ | N/A | 1093.13 | - |
| 132Te | 131.908553(7) | 3.204(13) d | ß- to 132I | 0+ | N/A | 1101.21 | - |
| 133Te | 132.910955(26) | 12.5(3) min | ß- to 133I | (3/2+) | N/A | 1099.97 | - |
| 134Te | 133.911369(11) | 41.8(8) min | ß- to 134I | 0+ | N/A | 1108.05 | - |
| 135Te | 134.91645(10) | 19.0(2) s | ß- to 135I | (7/2-) | N/A | 1116.12 | - |
| 136Te | 135.92010(5) | 17.63(8) s | ß- to 136I; ß- + n to 135I | 0+ | N/A | 1114.89 | - |
| 137Te | 136.92532(13) | 2.49(5) s | ß- to 137I; ß- + n to 136I | 3/2-# | N/A | 1122.97 | - |
| 138Te | 137.92922(22)# | 1.4(4) s | ß- to 138I; ß- + n to 137I | 0+ | N/A | 1131.04 | - |
| 139Te | 138.93473(43)# | 500# ms [>300 ns] | ß- to 139I; ß- + n to 138I | 5/2-# | N/A | 1129.81 | - |
| 140Te | 139.93885(32)# | 300# ms [>300 ns] | ß- to 140I; ß- + n to 139I | 0+ | N/A | 1137.89 | - |
| 141Te | 140.94465(43)# | 100# ms [>300 ns] | ß- to 141I; ß- + n to 140I | 5/2-# | N/A | 1136.65 | - |
| 142Te | 141.94908(64)# | 50# ms [>300 ns] | ß- to 142I | 0+ | N/A | 1144.73 | - |