About Molybdenum
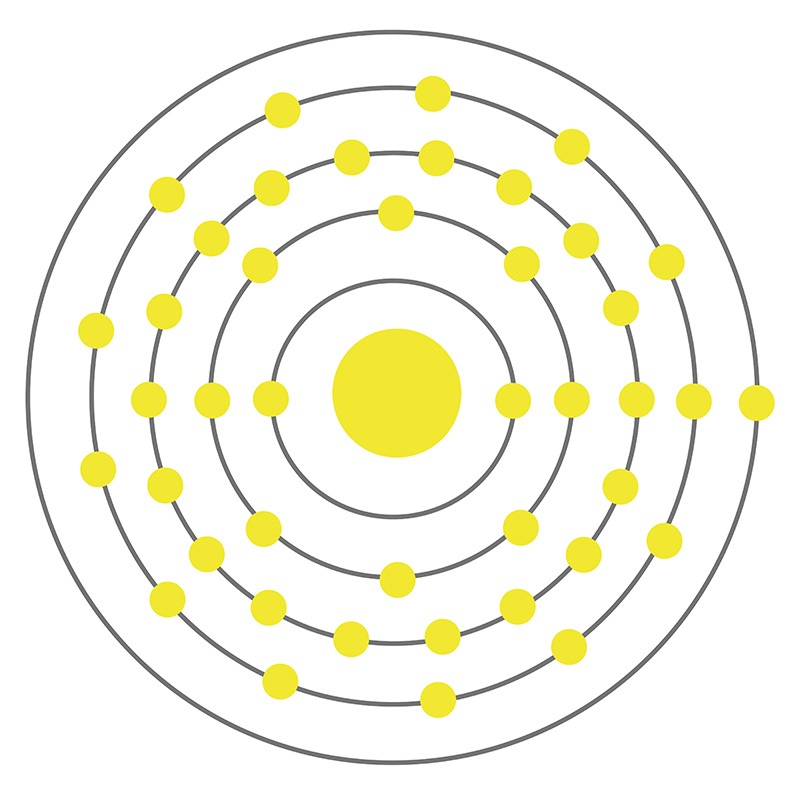
The chemical and physical properties of molybdenum metal and its alloys are prized by a wide range of industries ranging from defense, avionics, metallurgy, glassmaking, pigments and dyes, organometallic chemistry, and the manufacturing of photovoltaics and semiconductor devices. A silvery gray transition metal, molybdenum is also one of the refractory metals, a group of metals with the shared properties of extremely high melting points and thermal conductivity, low thermal expansion and vapor pressure, excellent dimensional stability and creep resistance, and resistance to oxidation; its chemical behavior is similar to that of tungsten. Molybdenum has the sixth highest melting point of all elements and one of the lowest coefficients of thermal expansion among commercial metals, yet possesses a density only 25% higher than iron. It is the 54th most abundant element in the earth’s crust but does not occur freely in nature, rather present in oxide forms with various valence states. Common molybdenum-containing minerals include molybdenite (molybdenum sulfide), wulfenite (lead molybdate), and powellite; the metal is commercially produced via the direct mining of molybdenite as well as the production of tungsten and copper.
Molybdenum-containing minerals have been known since ancient times, but the element was often identified as lead; its current name is derived from the Ancient Greek word for lead, molybdos, owing to the confusion between lead sulfide and molybdenum sulfides, the latter of which was originally referred to as molybdena. Carl Wilhelm Scheele identified the element in 1778 by producing molybdenum oxide from molybdenite (naming it “terra molybdaenae”); in 1781, Peter Jacob Hjelm heated linseed oil and carbon combined with molybdic acid to yield the metal.
Molybdenum is toxic in high amounts but plays a critical biological role as a cofactor required for enzyme function; it did not play a significant commercial role until utilized in German artillery during World War II. Since then, its role as an alloying agent has composed more than 75% of its usage; often referred to as “moly,” it lends numerous advantages including increased hardness, strength, creep resistance, resistance to wear and corrosion, weldability, and stability in high stress, high temperature environments. Besides quenched and tempered steels, prevalent molybdenum alloys used in x-ray tubes, forging tools, and other applications include Hastelloys, ferromolybdenum, Titanium-Zirconium-Molybdenum (TZM), and Molybdenum-Lanthanum (lanthanated moly, or MoLa) which exhibits higher strength and dimensional stability than TZM. Commercial products that benefit from the use of molybdenum include armor, aircraft engine components, glass melting electrodes, valves, boiler plates, ribbons and wires for lighting, semiconductor base plates, hot-zones and heat sinks, sputtering targets for photovoltaic cell coatings and flat screens, crucibles for sapphire growth, circuit inks, and microwave devices. The isotope Molybdenum-99 is also used in nuclear imaging.
Molybdenum compounds are often used as industrial catalysts for removal of sulfide compounds from crude oil, converting water to hydrogen gas, producing formaldehyde and acrylonitrile, and, in the case of the n-type semiconductor molybdenum trioxide, acting as a photocatalyst; other applications include lubricants and pigments. Molybdenum disulfide and molybdenum diselenide have the ability to form two-dimensional thin films analogous to those of carbon (in the form of graphene) for use in flexible electronics; electrodes composed of MoS2-graphene nanosheet composites vastly improve performance of next-generation sodium air batteries.
Products
Molybdenum is used in high-pressure and high-temperature environments as pigments and catalysts. It is also found used in nuclear reactors, aerospace components, and some forms of steel alloys to add hardness and raise melting points.  Molybdenum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).
Molybdenum is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity).  Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Molybdenum nanoparticles and nanopowders are also available. Molybdenum oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Molybdenum fluorides are other insoluble forms for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Molybdenum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Elemental or metallic forms include pellets, rod, wire and granules for evaporation source material purposes. Molybdenum nanoparticles and nanopowders are also available. Molybdenum oxides are available in powder and dense pellet form for such uses as optical coating and thin film applications. Oxides tend to be insoluble. Molybdenum fluorides are other insoluble forms for uses in which oxygen is undesirable such as metallurgy, chemical and physical vapor deposition and in some optical coatings. Molybdenum is also available in soluble forms including chlorides, nitrates and acetates. These compounds can be manufactured as solutions at specified stoichiometries.
Molybdenum Properties
 Molybdenum is a Block D, Group 6, Period 5 element.
Molybdenum is a Block D, Group 6, Period 5 element. 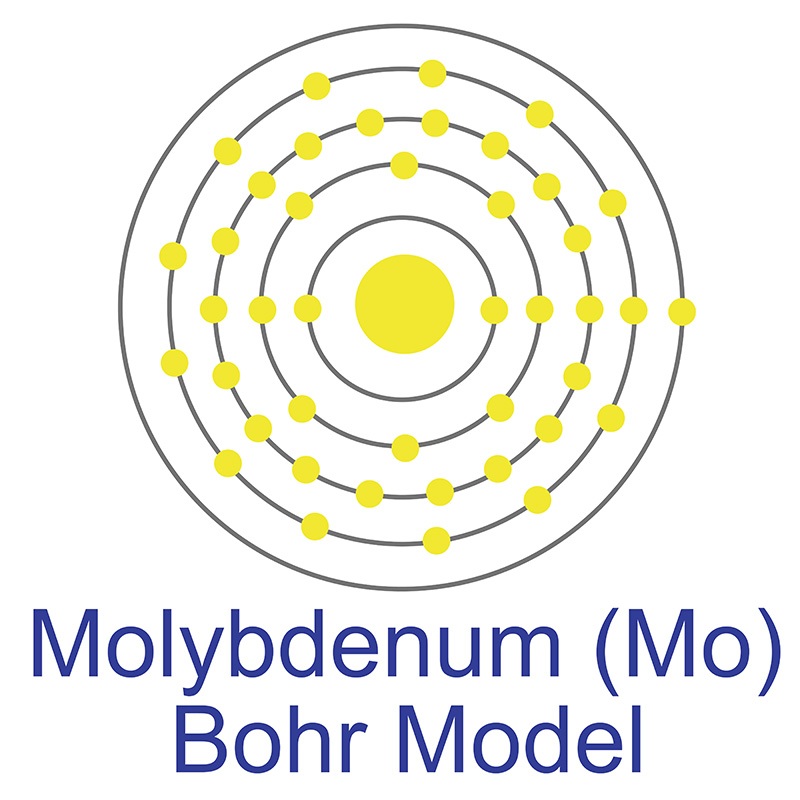 The number of electrons in each of molybdenum's shells is 2, 8, 18, 13, 1 and its electronic configuration is [Kr] 4d5 5s1. The molybdenum atom has a radius of 136.3.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7439-98-7, molybdenum has a gray metallic appearance.
The number of electrons in each of molybdenum's shells is 2, 8, 18, 13, 1 and its electronic configuration is [Kr] 4d5 5s1. The molybdenum atom has a radius of 136.3.pm and its Van der Waals radius is 200.pm. In its elemental form, CAS 7439-98-7, molybdenum has a gray metallic appearance.  Molybdenum is the 54th most abundant element in the earth's crust. It has the third highest melting point of any element, exceeded only by tungsten and tantalum. Molybdenum does not occur naturally as a free metal, it is found in various oxidation states in minerals. The primary commercial source of molybdenum is molybdenite, although it is also recovered as a byproduct of copper and tungsten mining. Molybdenum was first discovered by Carl Wilhelm in 1778. The origin of the name molybdenum comes from the Greek word molubdos meaning lead.
Molybdenum is the 54th most abundant element in the earth's crust. It has the third highest melting point of any element, exceeded only by tungsten and tantalum. Molybdenum does not occur naturally as a free metal, it is found in various oxidation states in minerals. The primary commercial source of molybdenum is molybdenite, although it is also recovered as a byproduct of copper and tungsten mining. Molybdenum was first discovered by Carl Wilhelm in 1778. The origin of the name molybdenum comes from the Greek word molubdos meaning lead.
Health, Safety & Transportation Information for Molybdenum
Molybdenum is toxic unless it is in small quantities. Safety data for Molybdenum and its compounds can vary widely depending on the form. For potential hazard information, toxicity, and road, sea and air transportation limitations, such as DOT Hazard Class, DOT Number, EU Number, NFPA Health rating and RTECS Class, please see the specific material or compound referenced in the Products tab. The below information applies to elemental (metallic) Molybdenum.
| Safety Data | |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228 |
| Hazard Codes | F |
| Risk Codes | 11 |
| Safety Precautions | 9-16-36/37/39 |
| RTECS Number | QA4680000 |
| Transport Information | UN 3089 4.1/PG 2 |
| WGK Germany | nwg |
| Globally Harmonized System of Classification and Labelling (GHS) |
|
Molybdenum Isotopes
Molybdenum has 5 stable isotopes:
| Nuclide | Isotopic Mass | Half-Life | Mode of Decay | Nuclear Spin | Magnetic Moment | Binding Energy (MeV) | Natural Abundance (% by atom) |
|---|---|---|---|---|---|---|---|
| 83Mo | 82.94874(54)# | 23(19) ms [6(+30-3) ms] | ß+ to 83Nb; ß+ + p to 82Zr | 3/2-# | N/A | 671.85 | - |
| 84Mo | 83.94009(43)# | 3.8(9) ms [3.7(+10-8) s] | ß+ to 84Nb | 0+ | N/A | 687.38 | - |
| 85Mo | 84.93655(30)# | 3.2(2) s | ß+ to 85Nb | (1/2-)# | N/A | 699.19 | - |
| 86Mo | 85.93070(47) | 19.6(11) s | ß+ to 86Nb | 0+ | N/A | 712.86 | - |
| 87Mo | 86.92733(24) | 14.05(23) s | ß+ to 87Nb; ß+ + p to 86Zr | 7/2+# | N/A | 723.73 | - |
| 88Mo | 87.921953(22) | 8.0(2) min | ß+ to 88Nb | 0+ | N/A | 737.4 | - |
| 89Mo | 88.919480(17) | 2.11(10) min | ß+ to 89Nb | (9/2+) | N/A | 747.34 | - |
| 90Mo | 89.913937(7) | 5.56(9) h | EC to 90Nb | 0+ | N/A | 761.01 | - |
| 91Mo | 90.911750(12) | 15.49(1) min | EC to 91Nb | 9/2+ | N/A | 770.95 | - |
| 92Mo | 91.906811(4) | Observationally Stable | - | 0+ | N/A | 783.69 | 14.84 |
| 93Mo | 92.906813(4) | 4.0(8)E+3 y | EC to 93Nb | 5/2+ | N/A | 791.77 | - |
| 94Mo | 93.9050883(21) | STABLE | - | 0+ | N/A | 800.78 | 9.25 |
| 95Mo | 94.9058421(21) | STABLE | - | 5/2+ | -0.9142 | 808.86 | 15.92 |
| 96Mo | 95.9046795(21) | STABLE | - | 0+ | N/A | 817.87 | 16.68 |
| 97Mo | 96.9060215(21) | STABLE | - | 5/2+ | -0.9335 | 824.08 | 9.55 |
| 98Mo | 97.9054082(21) | Observationally Stable | - | 0+ | N/A | 833.09 | 24.13 |
| 99Mo | 98.9077119(21) | 2.7489(6) d | ß- to 99Tc | 1/2+ | 0.375 | 839.31 | - |
| 100Mo | 99.907477(6) | 8.5(5)E+18 y | 2ß- to 100Ru | 0+ | N/A | 847.39 | 9.63 |
| 101Mo | 100.910347(6) | 14.61(3) min | ß- to 101Tc | 1/2+ | N/A | 852.67 | - |
| 102Mo | 101.910297(22) | 11.3(2) min | ß- to 102Tc | 0+ | N/A | 860.75 | - |
| 103Mo | 102.91321(7) | 67.5(15) s | ß- to 103Tc | (3/2+) | N/A | 868.83 | - |
| 104Mo | 103.91376(6) | 60(2) s | ß- to 104Tc | 0+ | N/A | 876.91 | - |
| 105Mo | 104.91697(8) | 35.6(16) s | ß- to 105Tc | (5/2-) | N/A | 884.98 | - |
| 106Mo | 105.918137(19) | 8.73(12) s | ß- to 106Tc | 0+ | N/A | 893.06 | - |
| 107Mo | 106.92169(17) | 3.5(5) s | ß- to 107Tc | (7/2-) | N/A | 891.83 | - |
| 108Mo | 107.92345(21)# | 1.09(2) s | ß- to 108Tc | 0+ | N/A | 899.9 | - |
| 109Mo | 108.92781(32)# | 0.53(6) s | ß- to 109Tc | (7/2-)# | N/A | 907.98 | - |
| 110Mo | 109.92973(43)# | 0.27(1) s | ß- to 110Tc; ß- + n to 109Tc | 0+ | N/A | 916.06 | - |
| 111Mo | 110.93441(43)# | 200# ms [>300 ns] | ß- to 111Tc | N/A | N/A | 914.82 | - |
| 112Mo | 111.93684(64)# | 150# ms [>300 ns] | ß- to 112Tc | 0+ | N/A | 922.9 | - |
| 113Mo | 112.94188(64)# | 100# ms [>300 ns] | ß- to 113Tc | N/A | N/A | 921.67 | - |
| 114Mo | 113.94492(75)# | 80# ms [>300 ns] | Unknown | 0+ | N/A | 929.74 | - |
| 115Mo | 114.95029(86)# | 60# ms [>300 ns] | Unknown | N/A | N/A | 928.51 | - |